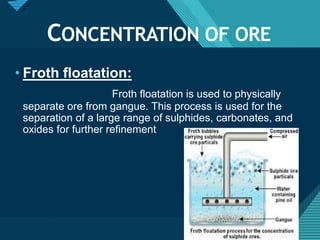
The document describes the process of refining copper from its ore. There are several key steps:
1) Mining the copper ore from underground deposits. 2) Crushing and grinding the ore to liberate copper particles from waste rock. 3) Concentrating the ore using froth flotation to separate copper minerals from waste gangue. 4) Extracting copper through roasting to form copper oxide and smelting to produce copper matte and remove iron. 5) Further refining copper using bessemerization and electrorefining or distillation to produce high purity copper.