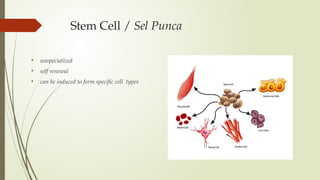
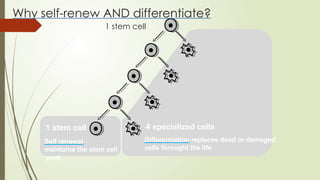
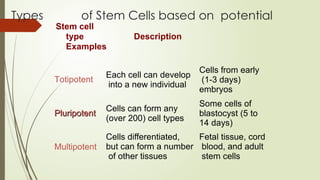
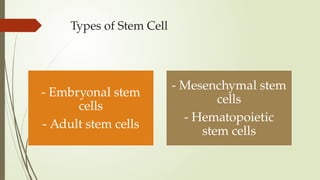
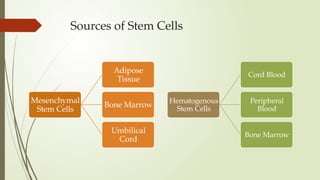
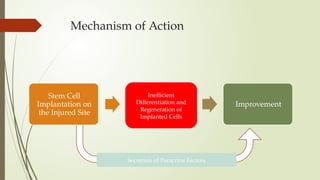
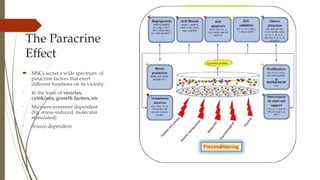
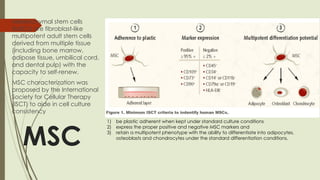
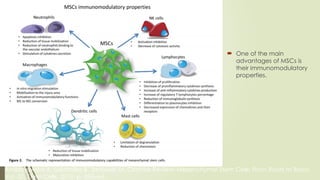
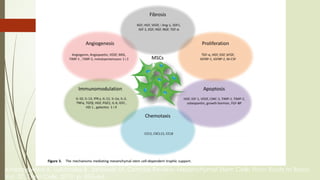
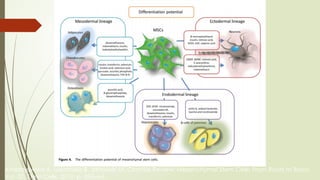
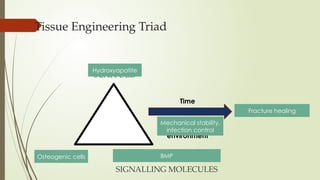
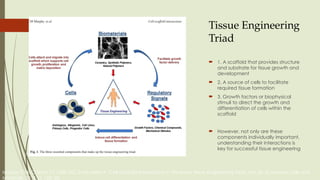
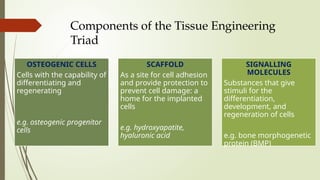
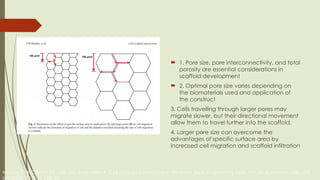
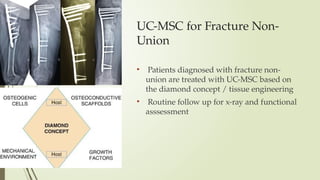
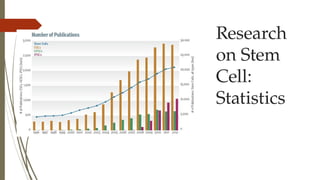
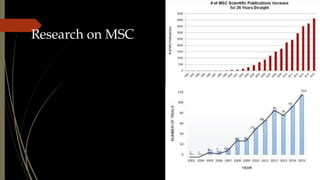
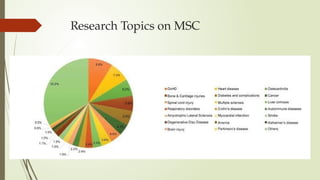
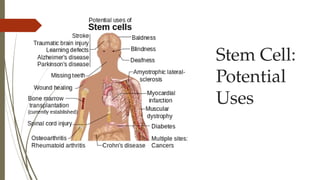
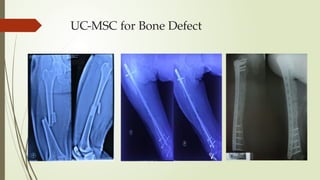
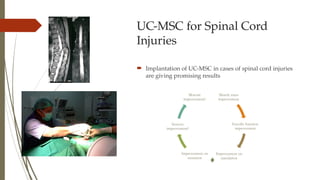
The document discusses stem cells, their types, properties, and applications in tissue renewal and regeneration, focusing on embryonic and adult stem cells. It elaborates on mesenchymal stem cells (MSCs), their sources, immunomodulatory advantages, and tissue engineering strategies, highlighting their role in treating conditions like knee osteoarthritis and spinal cord injuries. The conclusion emphasizes the promising outcomes of stem cell treatments while acknowledging the ongoing research and challenges in the field.