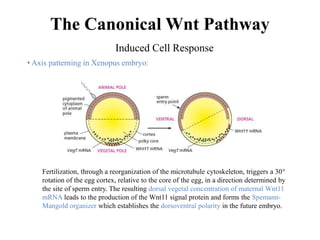
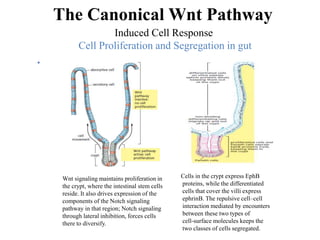
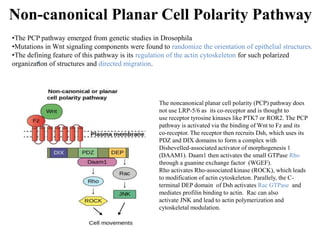
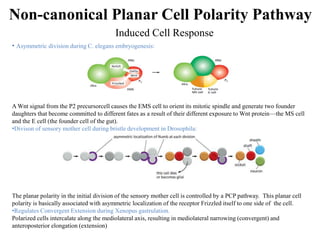
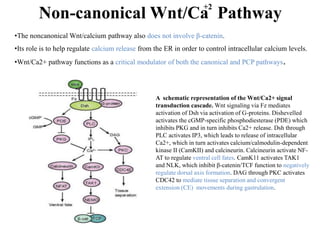
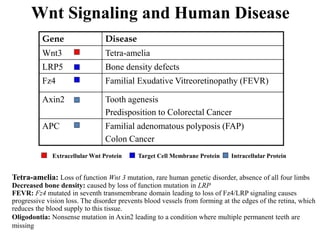
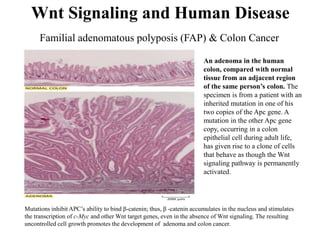
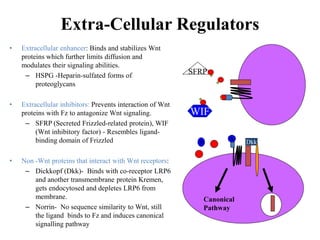
The document discusses the Wnt signaling pathway, a highly conserved mechanism critical for regulating cell fate, migration, and organogenesis during development, with implications for various diseases, including cancer. It details the components, mechanisms, and different Wnt pathways, including canonical and non-canonical pathways, and highlights the historical background of Wnt gene discovery. Additionally, it explores the role of Wnt signaling in human diseases and the associated genetic mutations impacting this pathway.

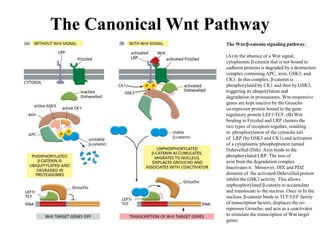
![The Canonical Wnt Pathway
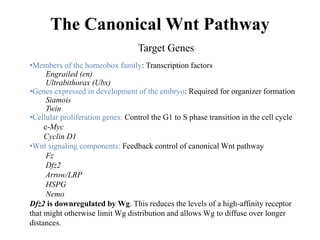
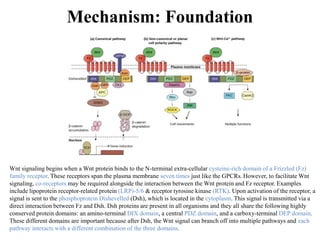
Nuclear Activity of β-Catenin. TCF provides sequence-specific binding activity and, in the absence of nuclear β-catenin,
partners with the transcriptional repressor Groucho and histone deacetylases to form a repressive complex. When β-catenin enters
the nucleus, it directly replaces Groucho and converts the complex to a transcriptional activator, thereby effecting the
transcription of Wnt target genes. Other members of this activating complex are the histone acetylase CBP/p300, and the
SWI/SNF complex member Brg-1. Lgs and Pygo also bind to β-catenin, possibly driving its nuclear localization. Negative
regulation of signaling is provided by NLK (Nemo-like kinase: which phosphorylates TCF, sending it to the cytoplasm], and
ICAT (inhibitor of catenin: disassociates TCF/ β-catenin-CBP complex) and Chibby, which are antagonists of β-catenin. In
addition to TCF, two other DNA-binding proteins have been shown to associate with β-catenin: Pitx2 and Prop1. In the case of
Prop1, β-catenin can act as a transcriptional activator or repressor of specific genes, depending on the co-factors present.
However, the participation of any particular β-catenin complex in transcriptional regulation is highly cell type-dependent.](https://image.slidesharecdn.com/wntsignaling-170401100244/85/Regulation-by-Wnt-Signaling-8-320.jpg)