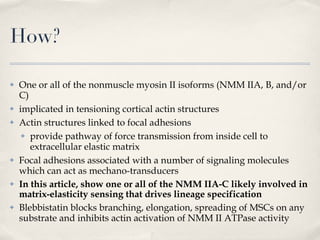
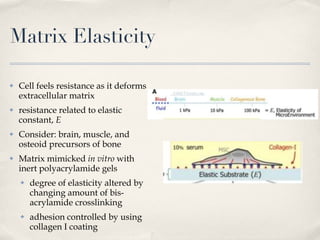
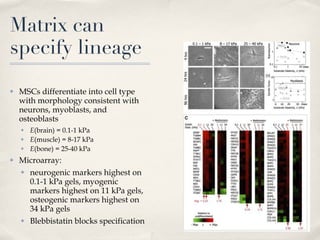
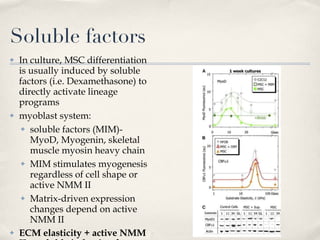
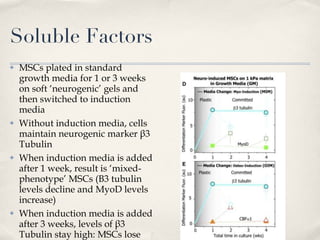
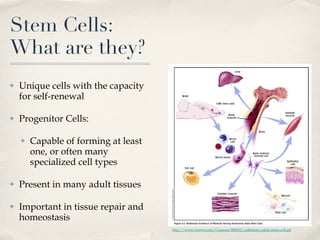
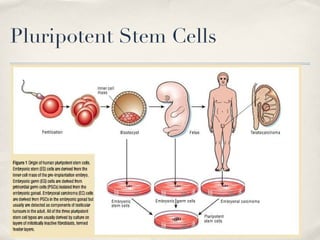
The document discusses different types of stem cells and how the elasticity of the extracellular matrix can influence stem cell differentiation. It finds that mesenchymal stem cells cultured on matrices of varying elasticity corresponding to brain, muscle, and bone tissues preferentially differentiate into neuron-like, myoblast-like, and osteoblast-like cells respectively. The stem cells appear to sense and respond to matrix stiffness through focal adhesions and actin-myosin contractility. Controlling the microenvironment may help direct stem cell differentiation for therapeutic applications.




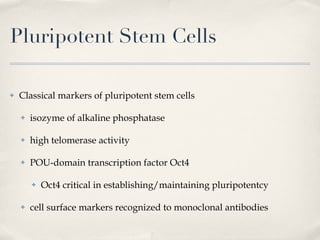
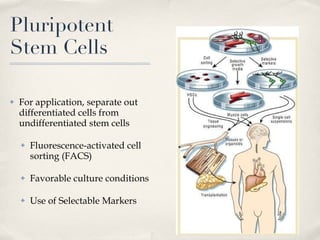



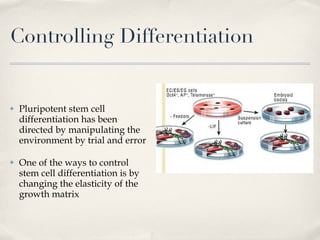


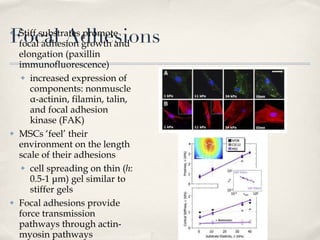
![Effect of matrix elasticity [...] Tissue-level matrix stiffness is distinct and shown here in sparse cultures to exert very strong effects on the lineage specification and commitment of naïve MSCs, as evident in cell morphology, transcript profiles, marker proteins, and the stability of responses How do the MSCs sense matrix elasticity? Ability to pull against matrix Requirement of cellular mechano-transducer to generate signal based on force Mechanotransduction of endothelial shear stress ( http://content.onlinejacc.org/cgi/content-nw/full/j.jacc.2007.02.059v1/FIG4 )](https://image.slidesharecdn.com/scdiferenciacinpormatriz-091111063128-phpapp02/85/S-CdiferenciacioNpormatriz-18-320.jpg)