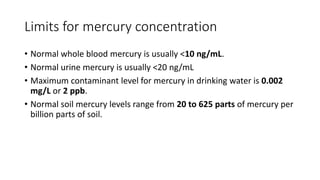
The document discusses mercury, its sources and effects on human health. Mercury occurs naturally and is released into the environment through volcanic activity, coal burning and mining. It can accumulate in fish and shellfish consumed by humans. Exposure to mercury, even in small amounts, can harm the nervous, digestive and immune systems. Methylmercury exposure from eating contaminated fish poses the greatest health risk. Preventive measures include promoting cleaner energy to reduce coal burning and phasing out non-essential mercury-containing products.