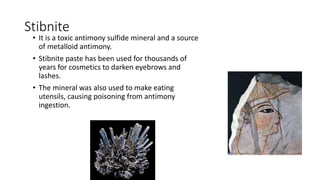
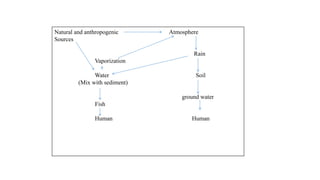
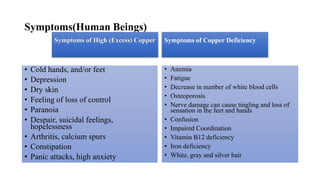
Selenium is an essential element found naturally in the environment through weathering of rocks. It enters plants and air through dust and is released during combustion of coal and oil. Both too little and too much selenium can impact human health. It can accumulate in organisms and biomagnify up the food chain. Occupational exposure to high levels of selenium through air can cause respiratory effects. Measures are needed to reduce selenium levels to protect environmental and human health.