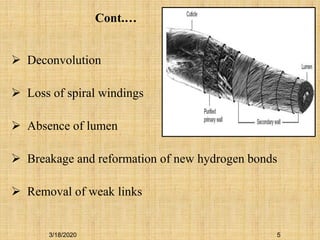
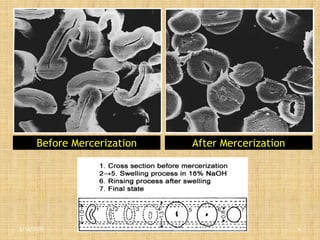
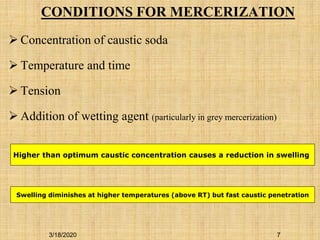
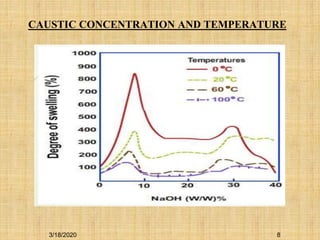
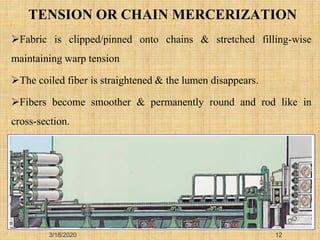
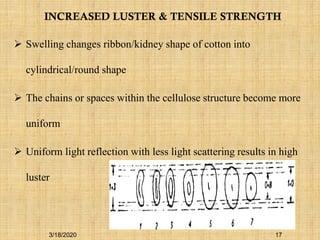
Mercerization is a treatment of cotton fabric with a concentrated solution of sodium hydroxide (NaOH). This process results in swelling and structural modification of the cotton fibers. Specifically, mercerization breaks hydrogen bonds in cellulose and allows molecular chains to rearrange, forming new bonds. This leads to increased luster, tensile strength, dye uptake, and dimensional stability of the cotton fabric. Conventionally, mercerization uses 18-24% NaOH solution at 15-18°C for 55 seconds while applying tension to the fabric. The process involves impregnation in the alkaline solution, stabilization, rinsing, and drying stages.













![STAGES OF MERCERIZATION
Mercerization can be carried out at different stages:
Grey mercerization [Addition of wetting agents]
After Desizing
After scouring [Sufficient washing off of caustic liquor]
After bleaching
Mercerization before bleaching can be disadvantage due to
contamination of caustic recovery --- Problem in caustic recovery
Mercerization after bleaching can also be a disadvantage -
Tendency of yellowness
3/18/2020 21](https://image.slidesharecdn.com/mercerizationlecture7-210208122322/85/Mercerization-lecture-7-21-320.jpg)





