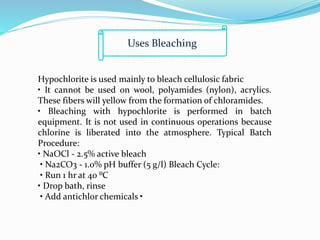
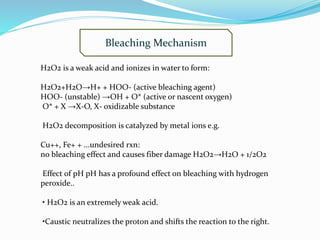
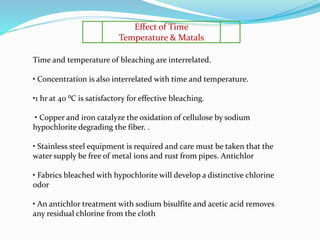
The document discusses bleaching, which involves decolorizing fabrics using oxidizing agents to break down color-producing compounds. It describes the mechanisms of bleaching using oxidative agents like hypochlorite and hydrogen peroxide. Key factors that affect bleaching like pH, time, temperature, and metal ions are outlined. Typical bleaching procedures and recipes for cotton, viscose, and other fibers are provided. Advantages of chlorine dioxide bleaching include complete removal of impurities and a high whiteness, while disadvantages include pollution from ClO2 and the need for acidic conditions.