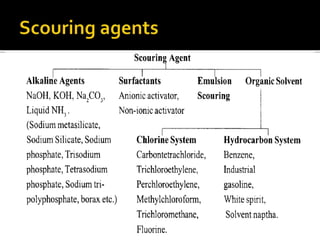
Scouring is a process that removes natural and added impurities from textiles through the use of chemicals like alkalis and surfactants. It saponifies fats and waxes, breaks down proteins and pectins, and increases the textile's absorptive capacity. The type of scouring agent used depends on the fiber and extent of impurities. Scouring emulsifies and saponifies impurities to solubilize and suspend them for removal, improving dyeability and finish performance.