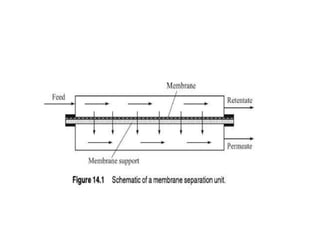
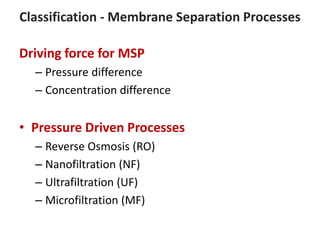
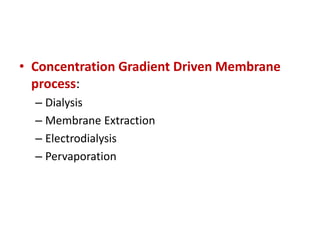
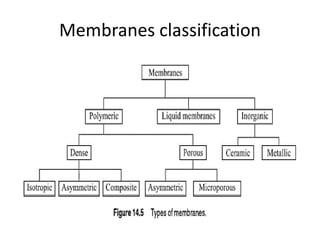
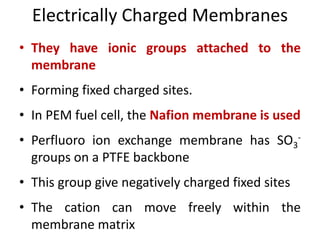
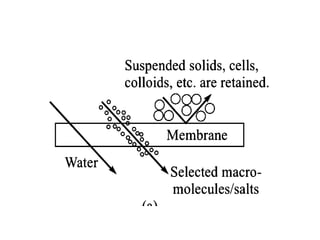
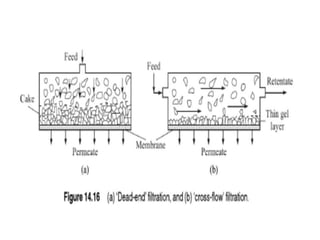
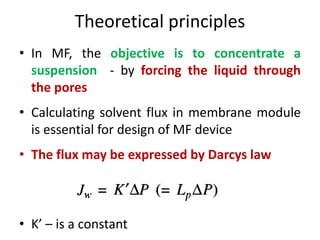
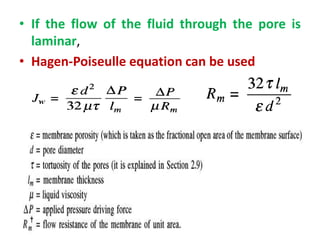
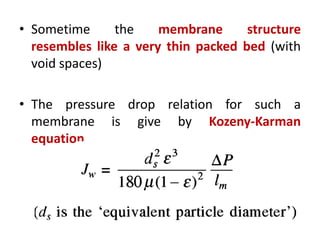
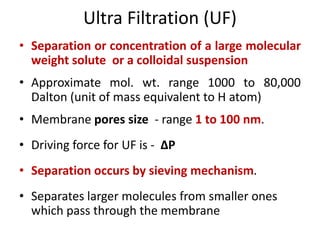
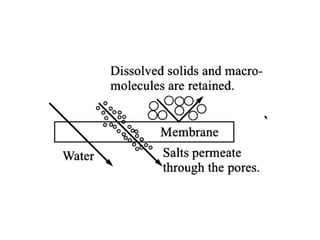
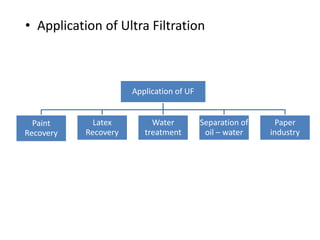
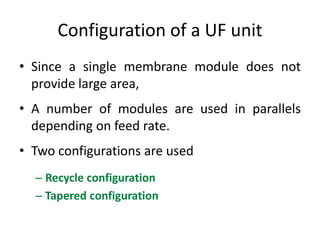
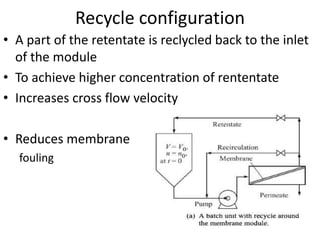
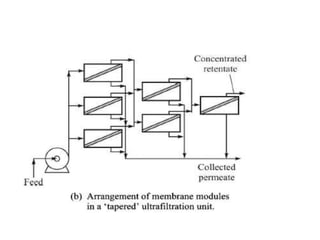
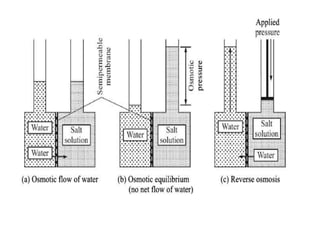
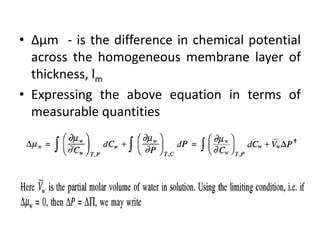
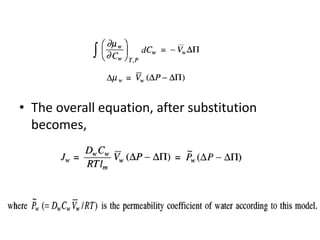
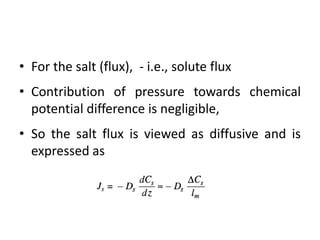
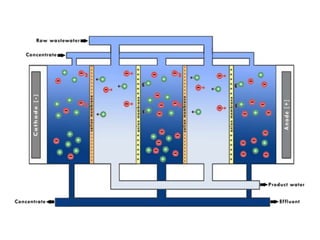
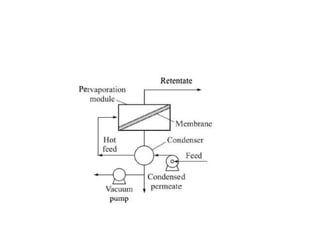
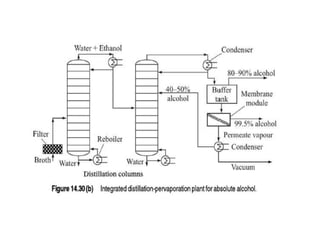
This document provides an overview of membrane separation processes. It discusses different types of membranes based on their structure like dense/non-porous, porous, asymmetric, composite and electrically charged membranes. It also describes various membrane separation techniques like microfiltration, ultrafiltration, reverse osmosis, dialysis, electrodialysis and pervaporation. Key applications and theoretical principles of each process are outlined. The document provides a comprehensive introduction to membrane separation technology.