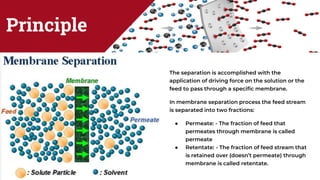
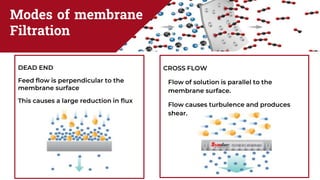
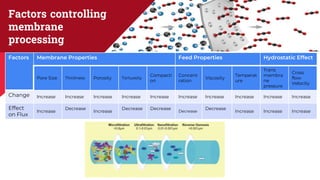
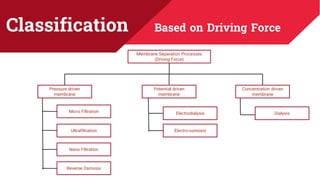
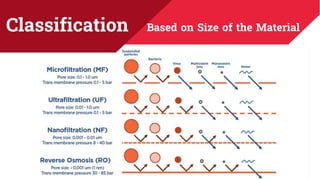
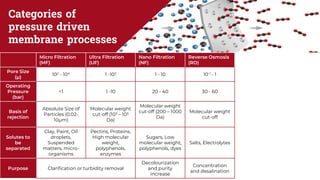
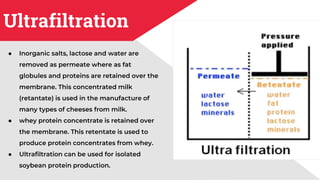
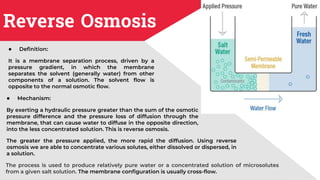
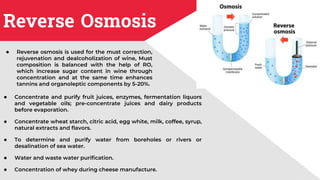
The document discusses membrane separation processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, which are widely used in chemical and food industries for various separation tasks. Key principles, types of membranes, processing modes, advantages, and applications in food processing are outlined, highlighting their efficiency and environmental benefits. The document concludes that membrane technology is a green alternative to traditional methods, driving the innovation of new food products.