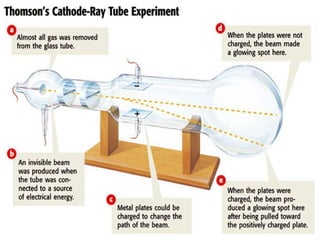
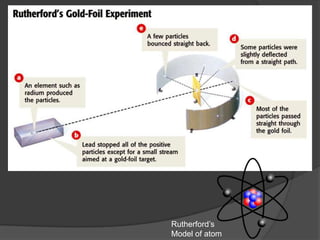
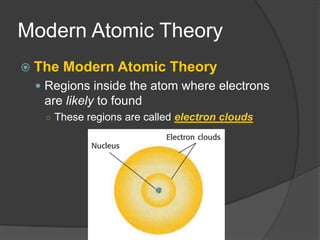
Democritus was one of the first to propose the idea of atoms in 440 BCE, suggesting that matter is made up of tiny indivisible particles called atoms. In the early 1900s, experiments led scientists to develop more accurate models of the atom. Rutherford discovered the nucleus in 1909 by firing positively charged particles at a gold foil and observing some particles deflecting, indicating a small, dense nucleus. Bohr then proposed in 1913 that electrons orbit the nucleus in distinct energy levels. The modern atomic theory describes the regions where electrons are likely to be found as electron clouds.