- The Grotthuss-Draper and Stark-Einstein laws state that light must be absorbed by a chemical system for a photochemical reaction to occur and that each photon activates only one molecule.
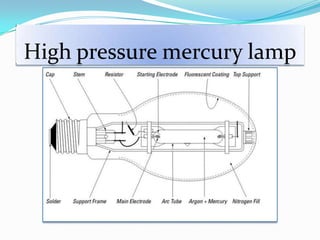
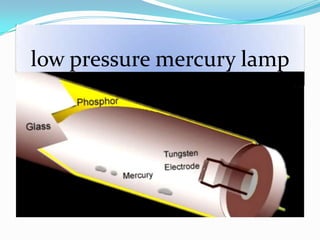
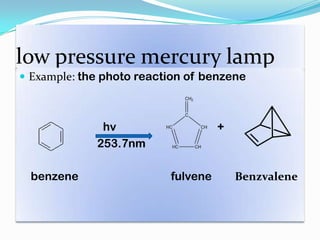
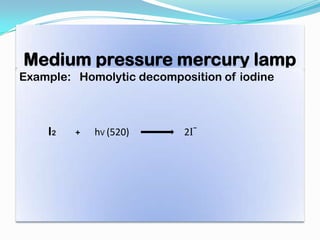
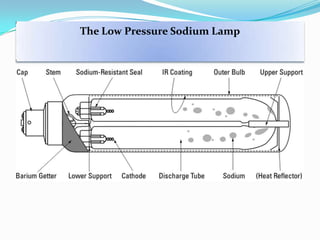
- Common light sources used in photochemistry include the sun, mercury lamps, halogen lamps, lasers, and light emitting diodes. Mercury lamps operate at different pressures which impact their properties.
- The type of light source used depends on the desired wavelength range and application. Each light source has advantages and disadvantages for photochemical reactions and experiments.