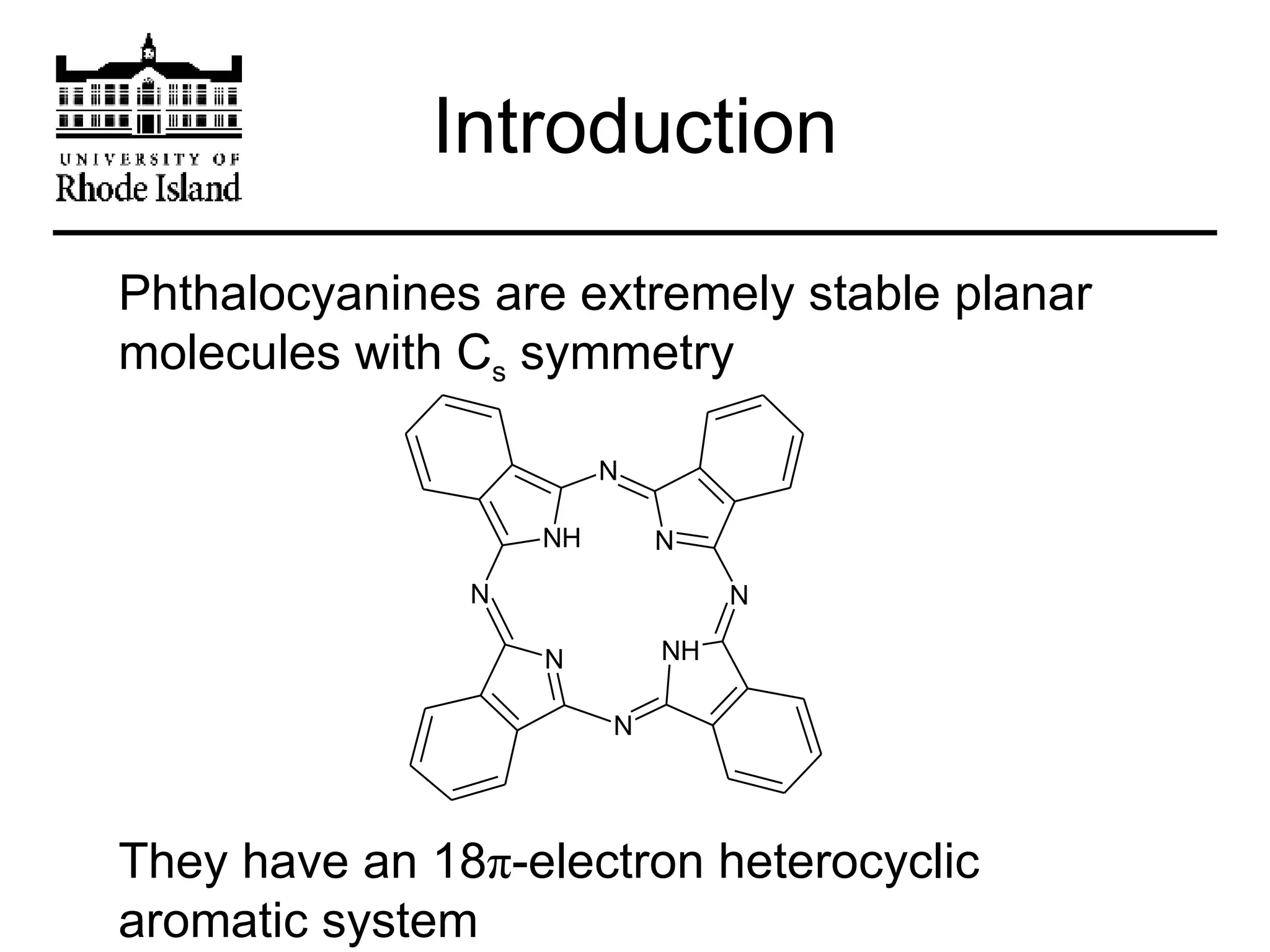
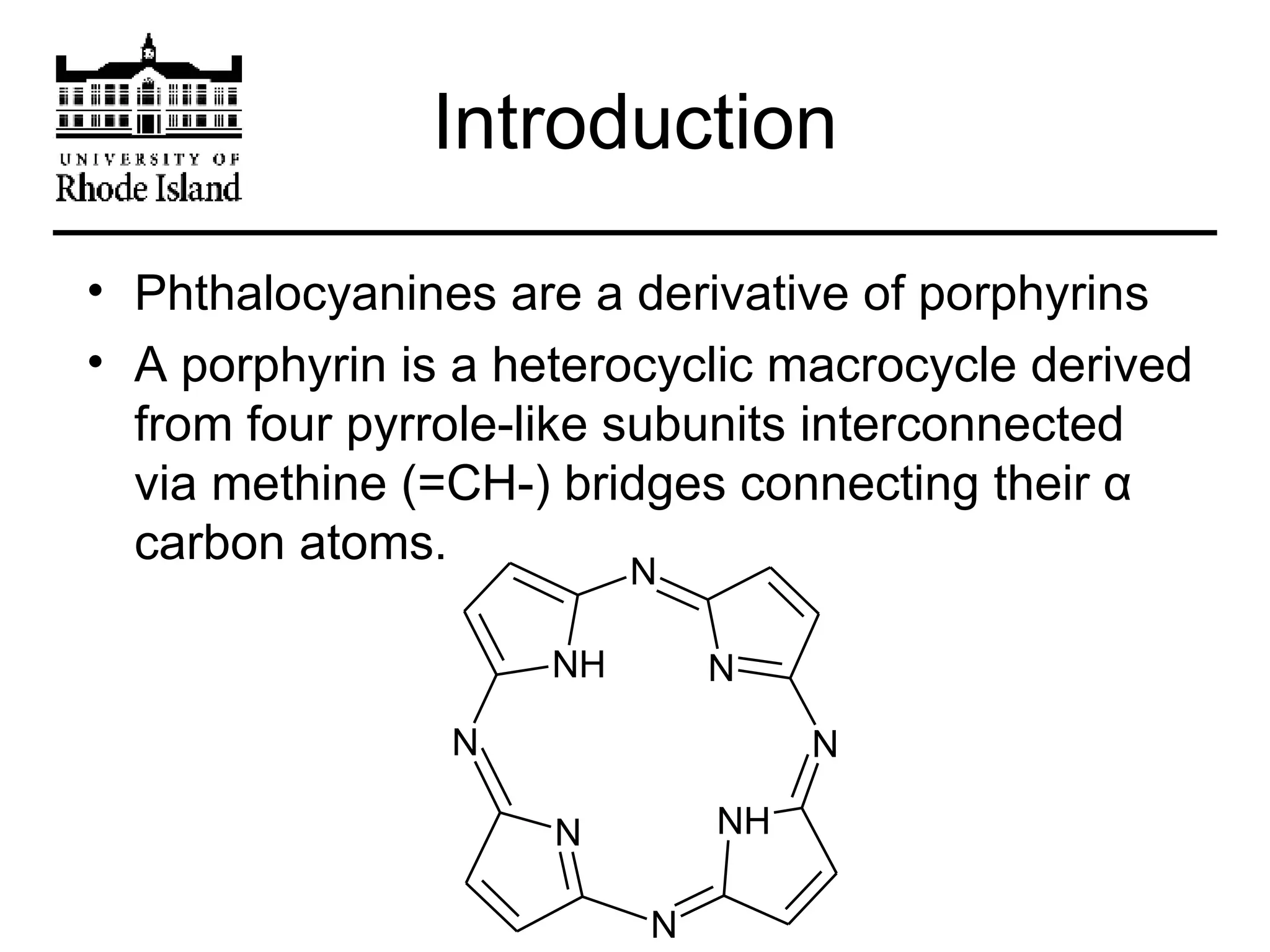
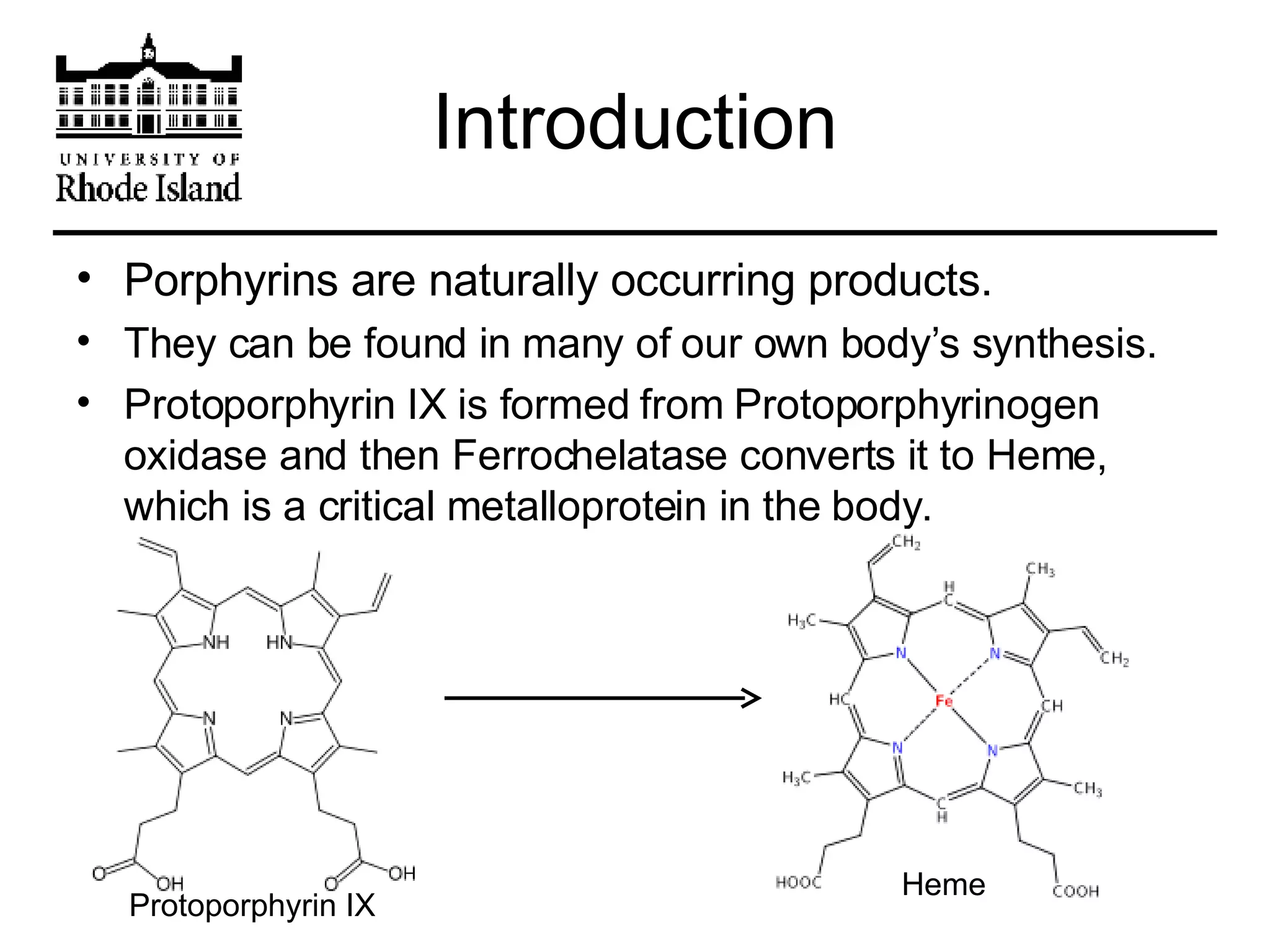
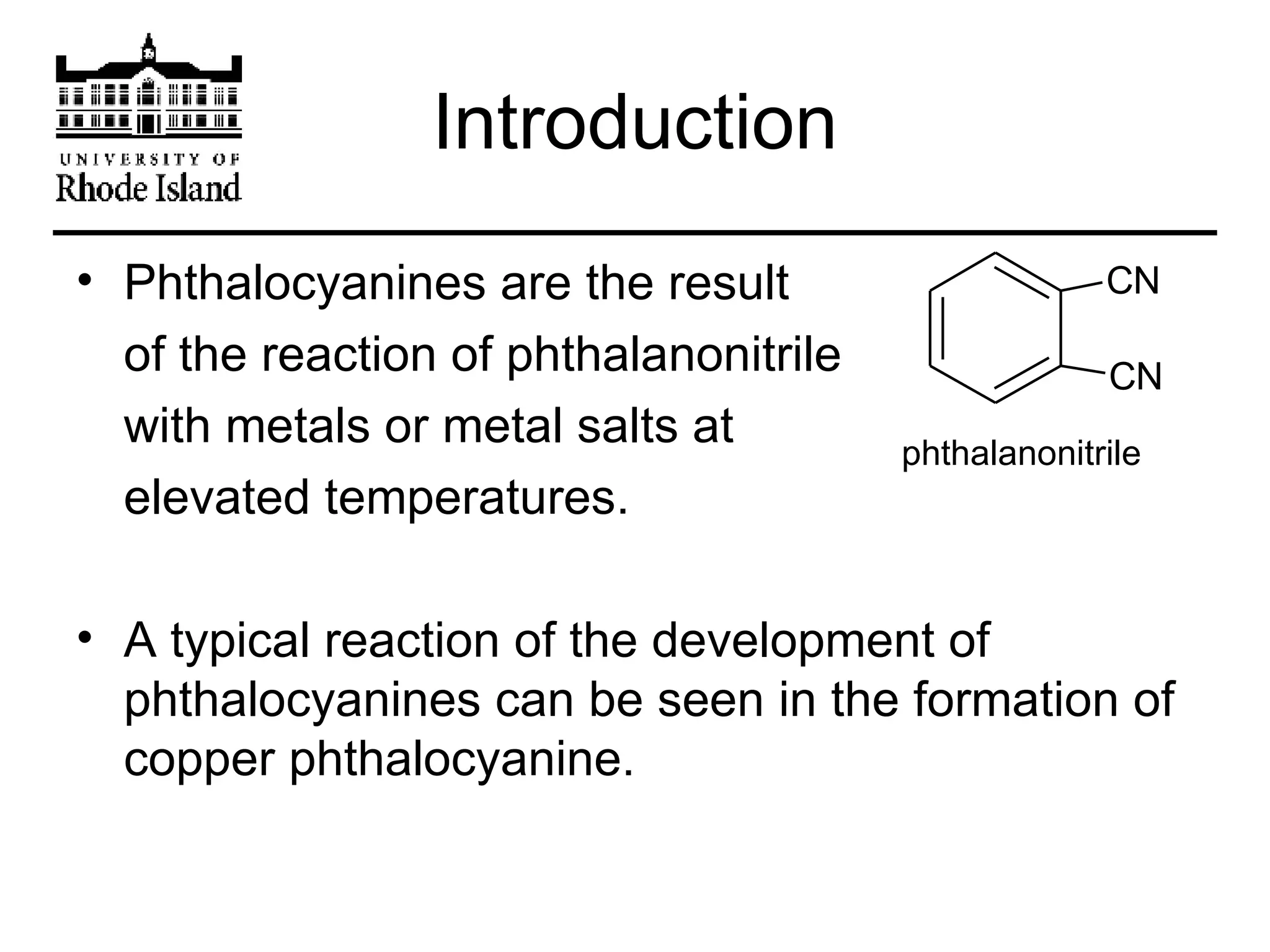
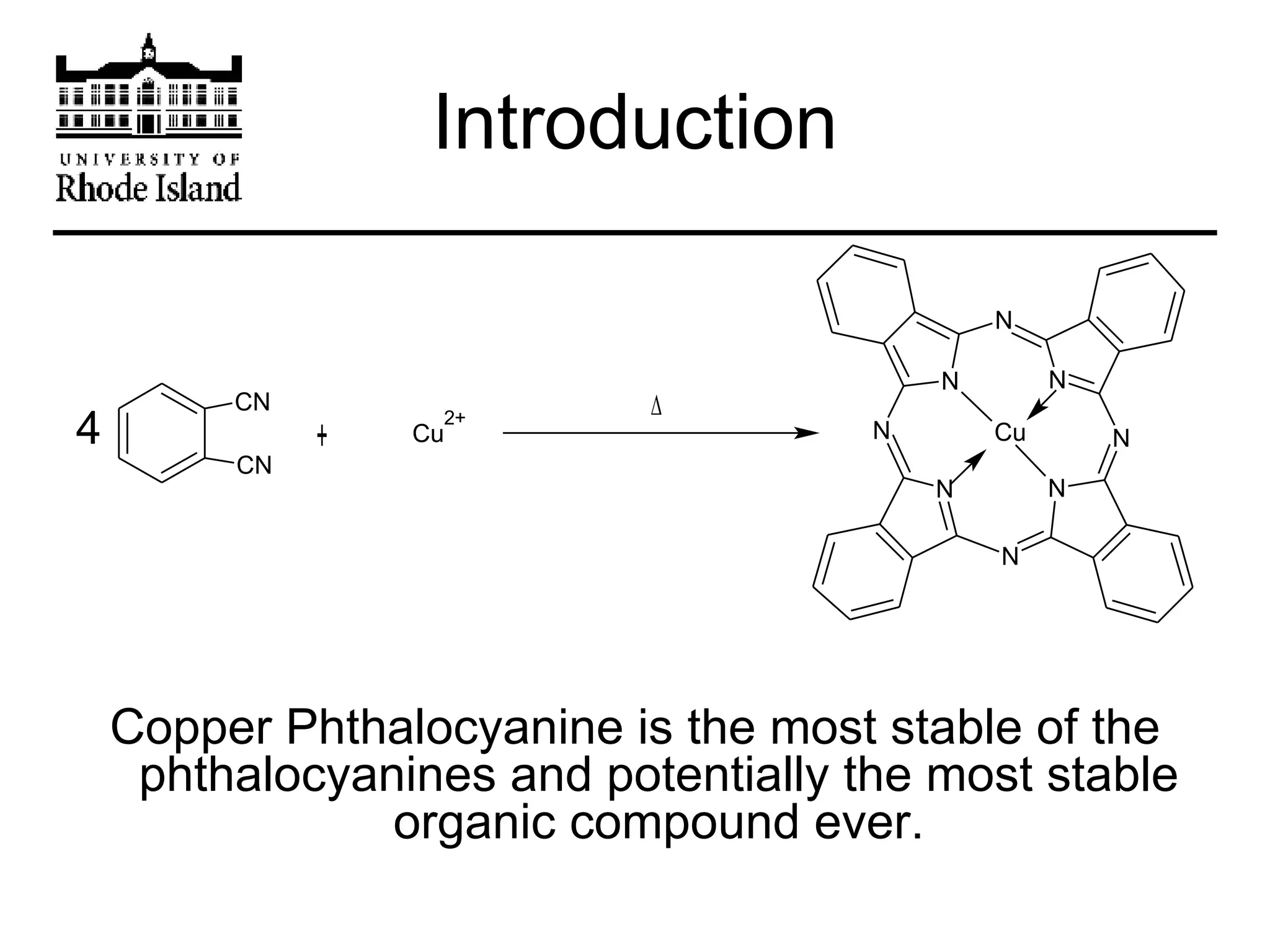
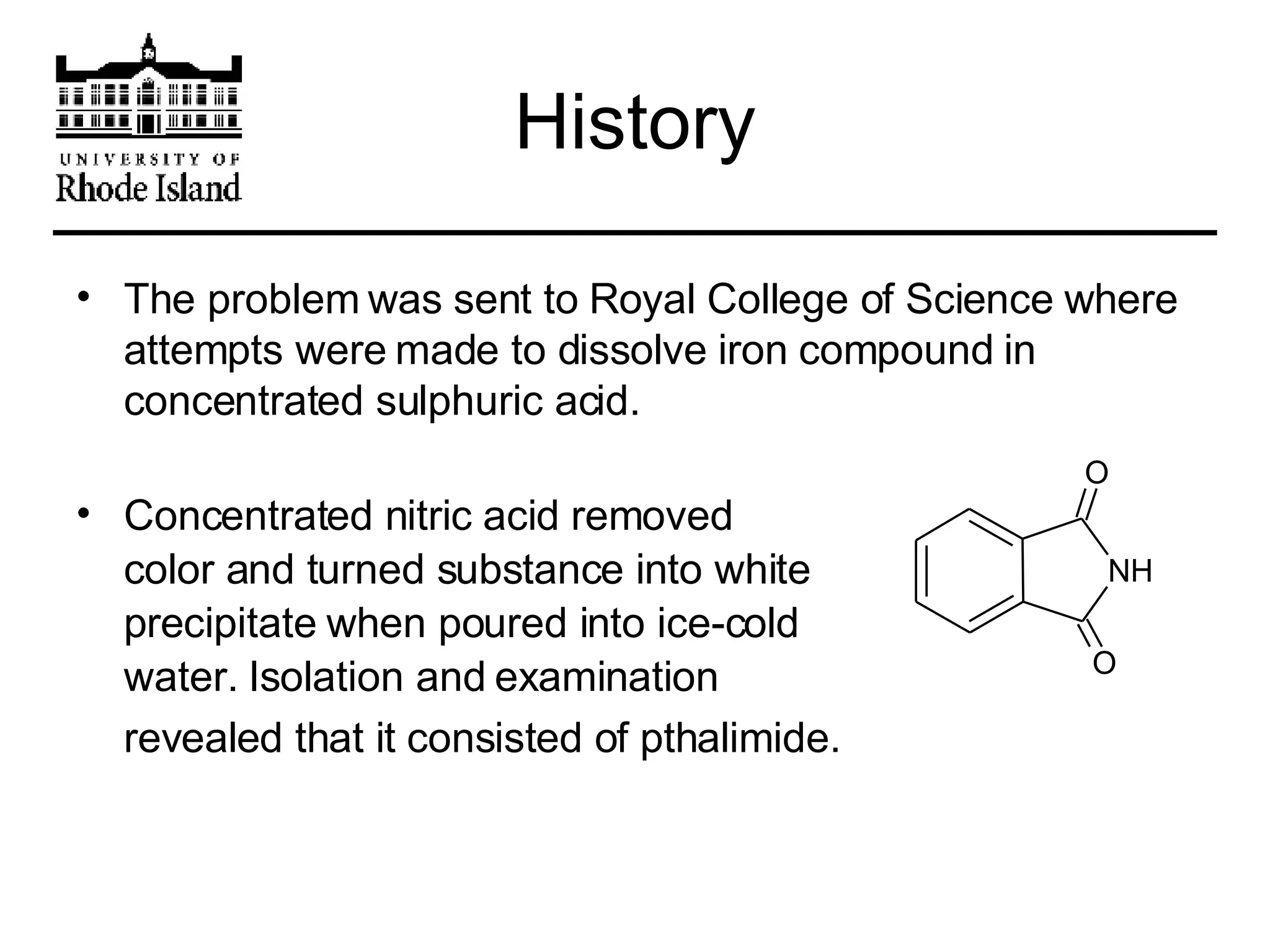
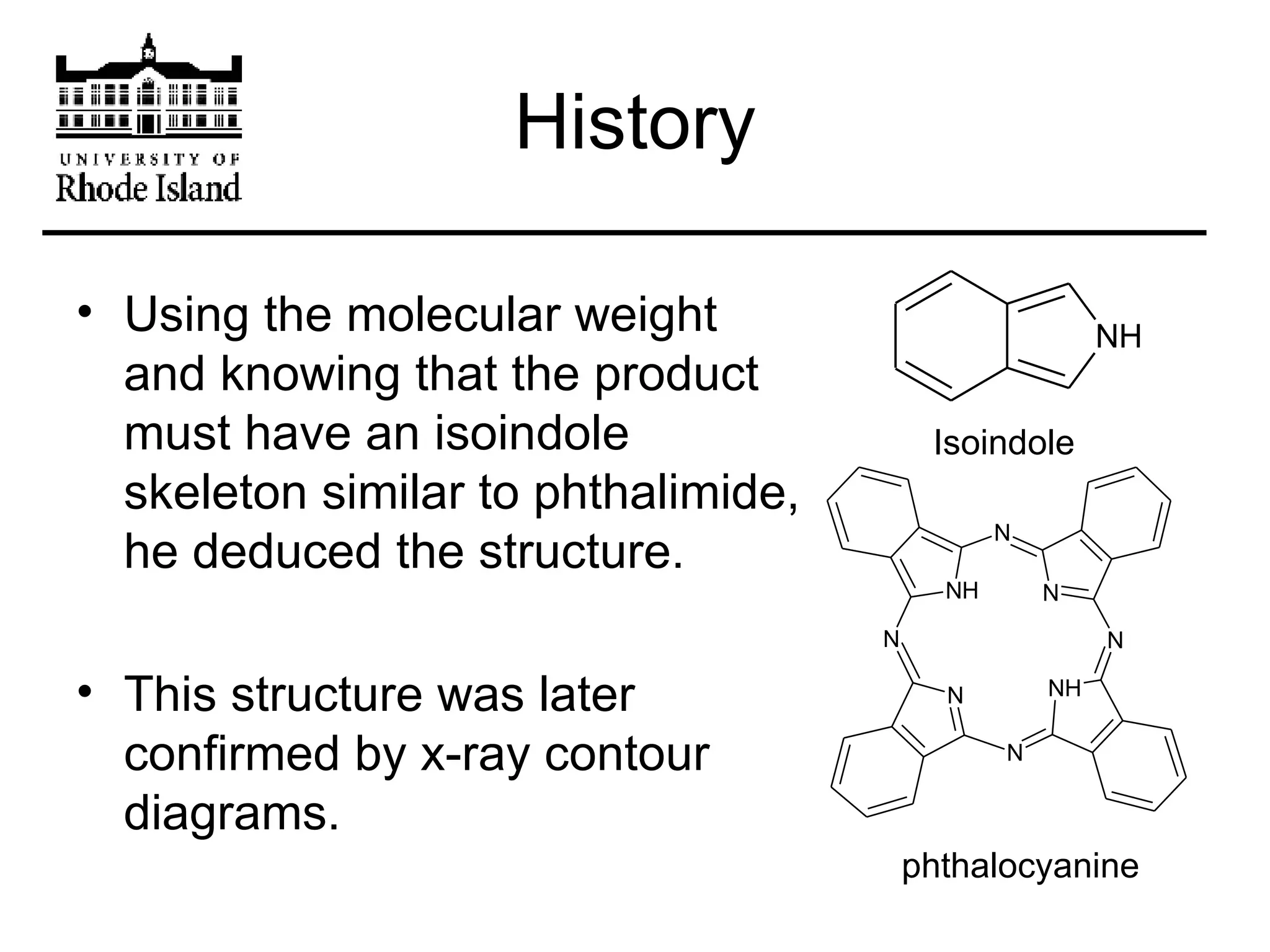
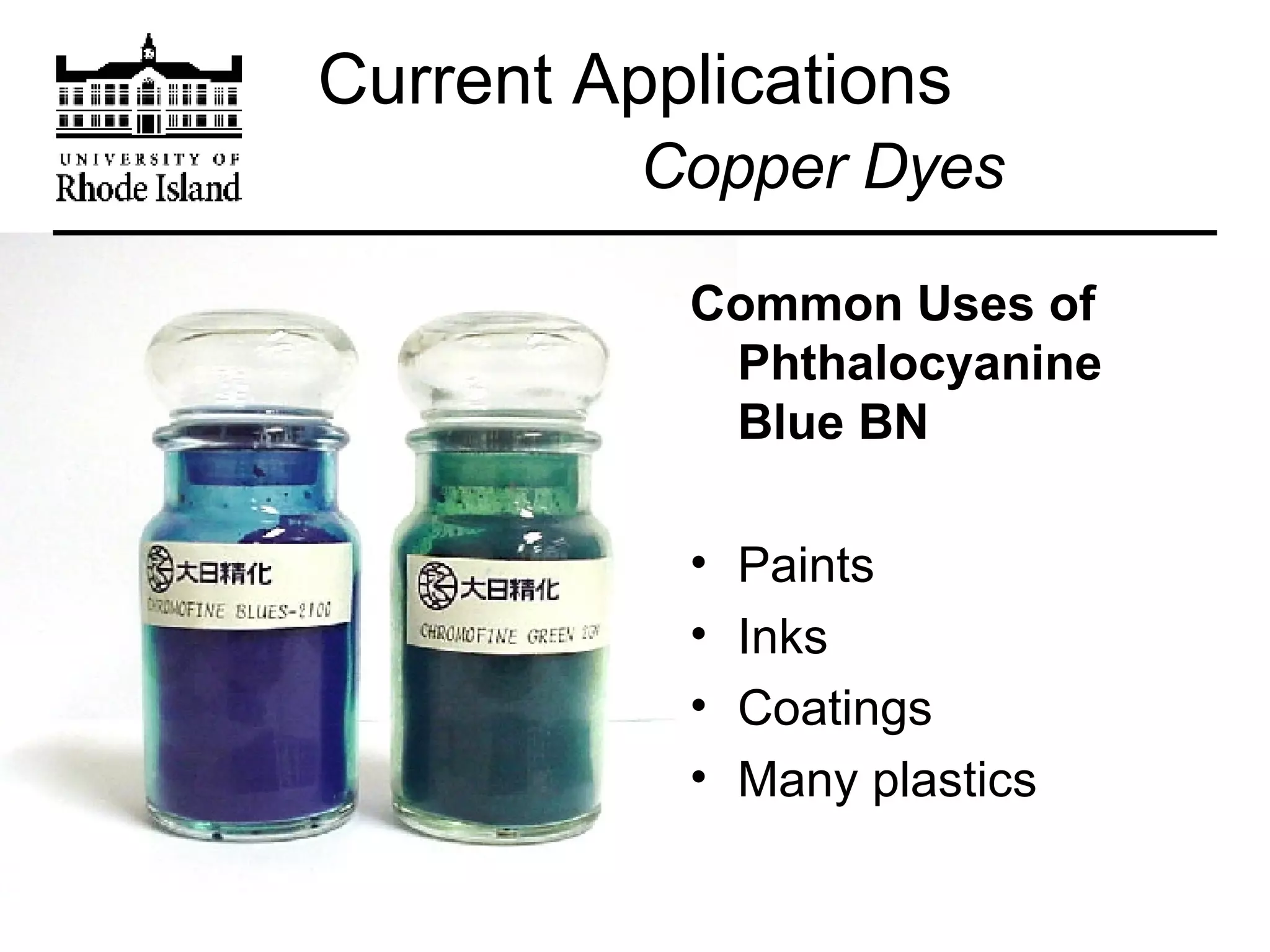
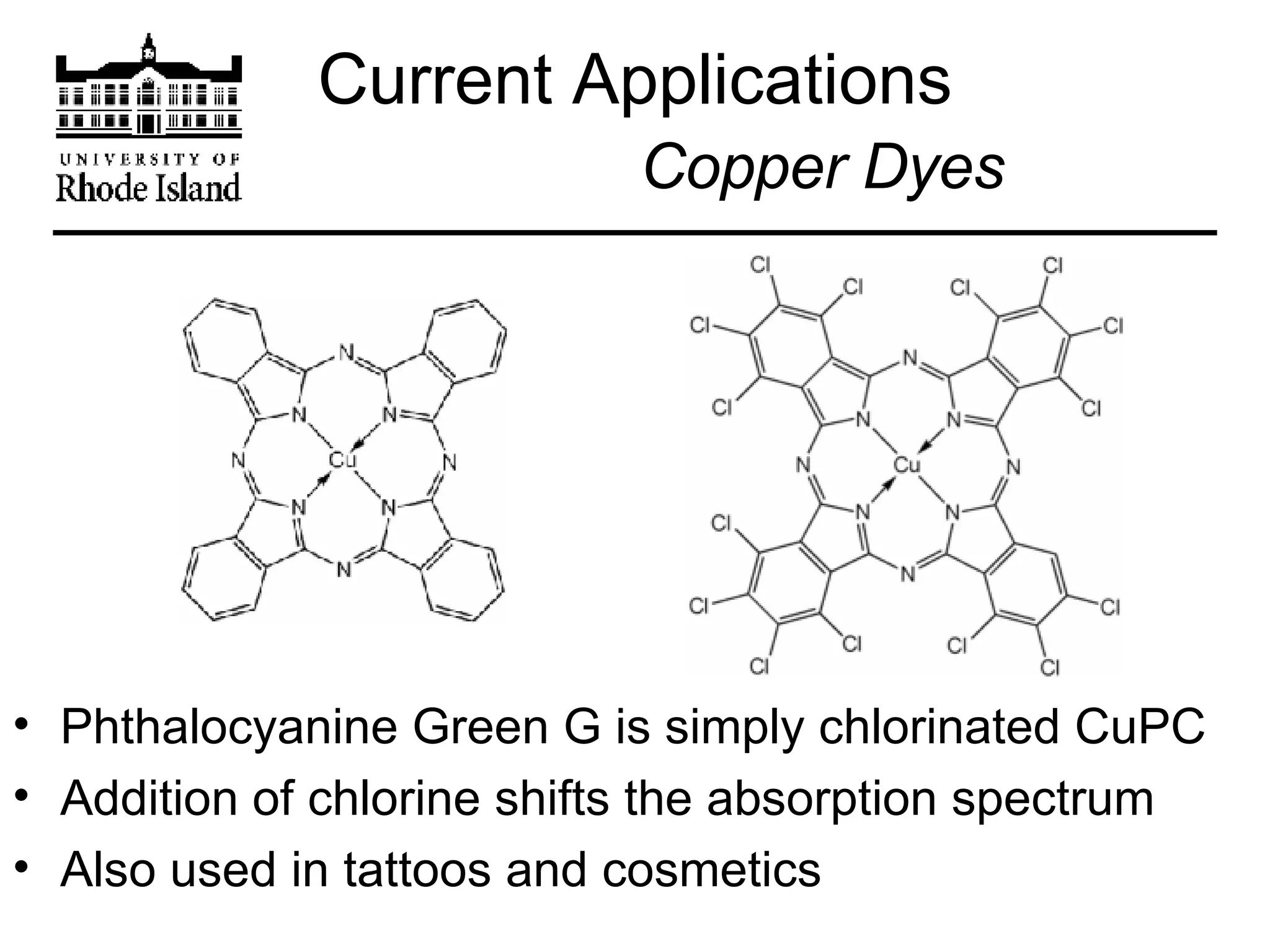
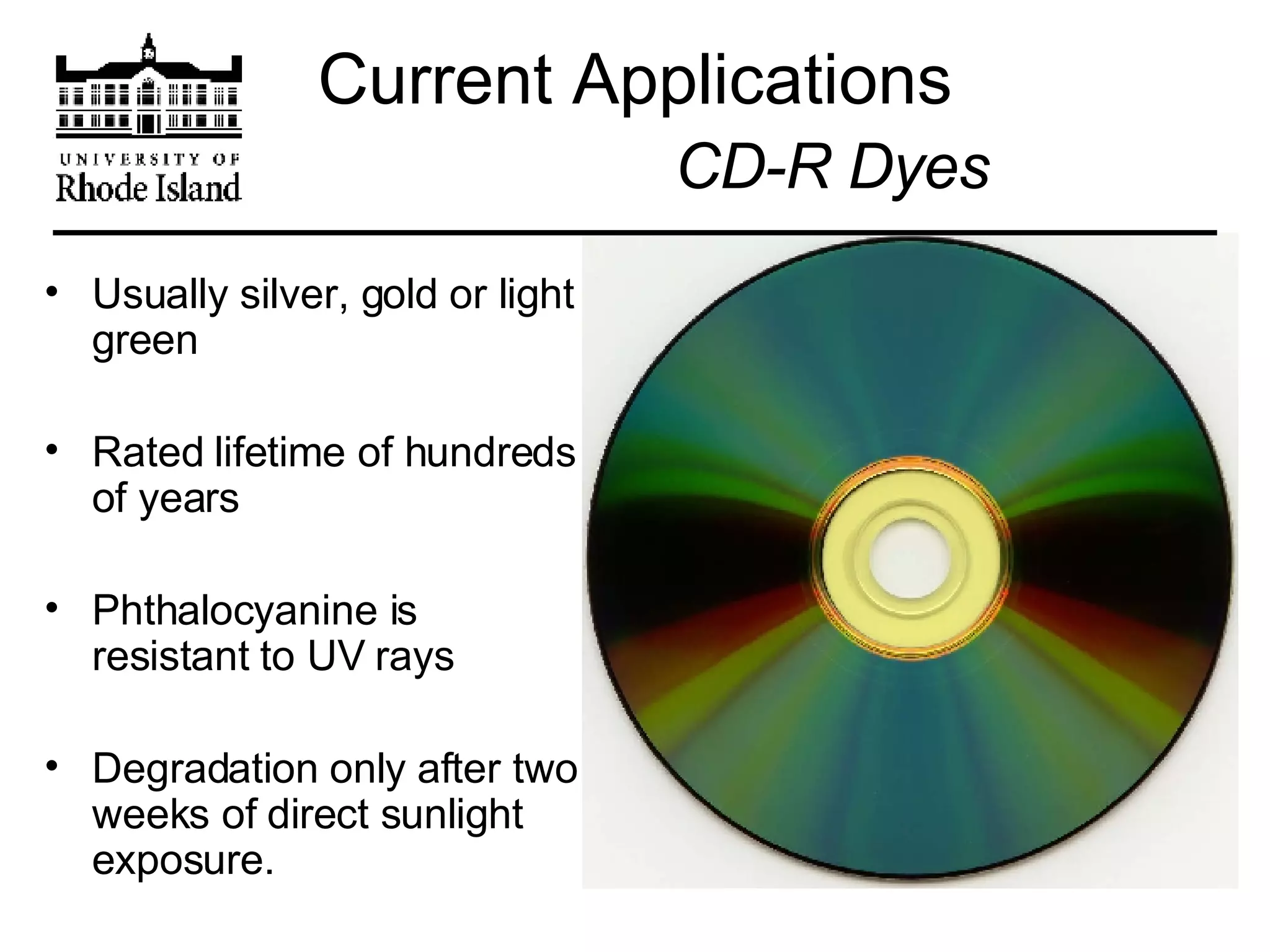
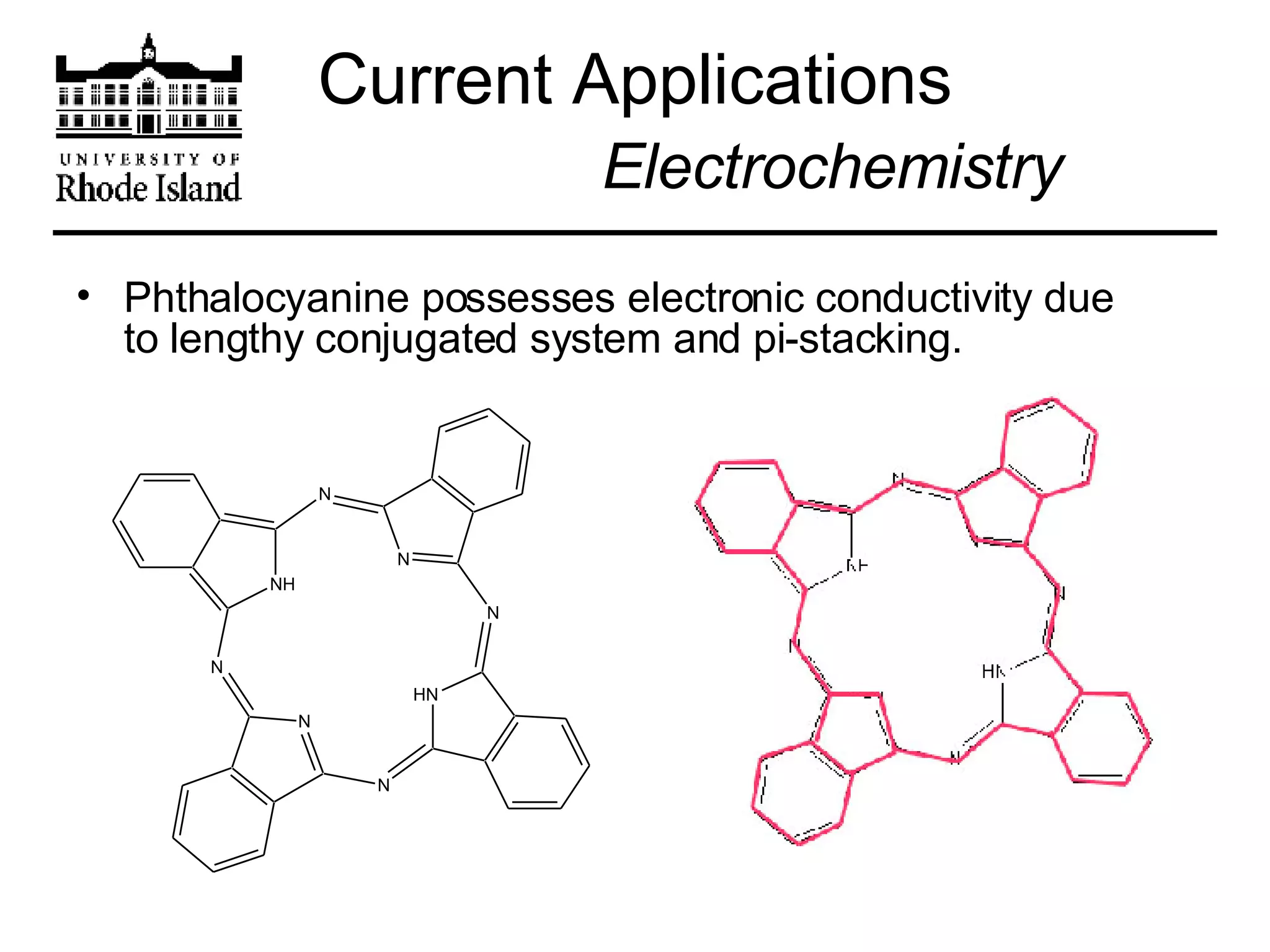
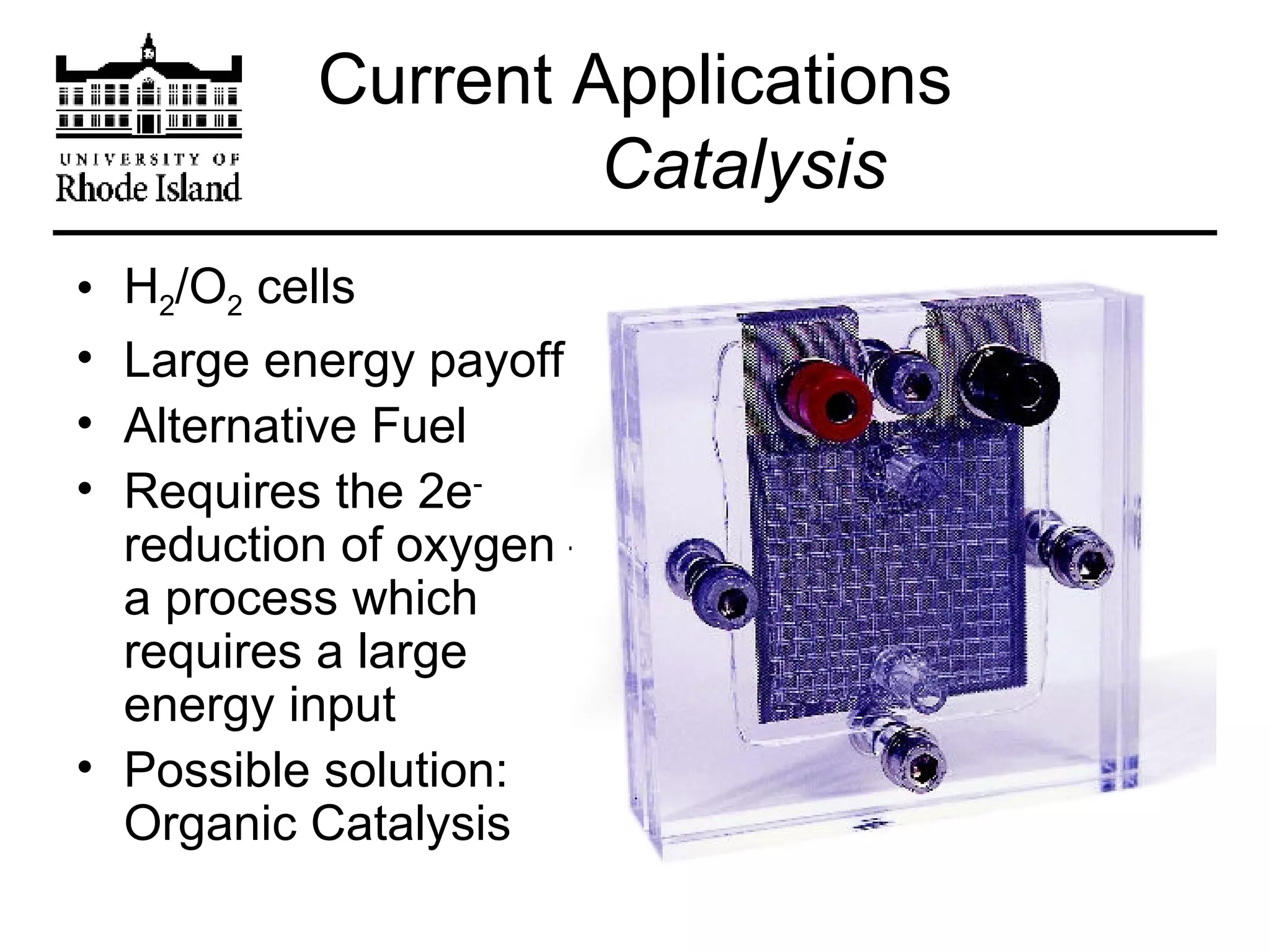
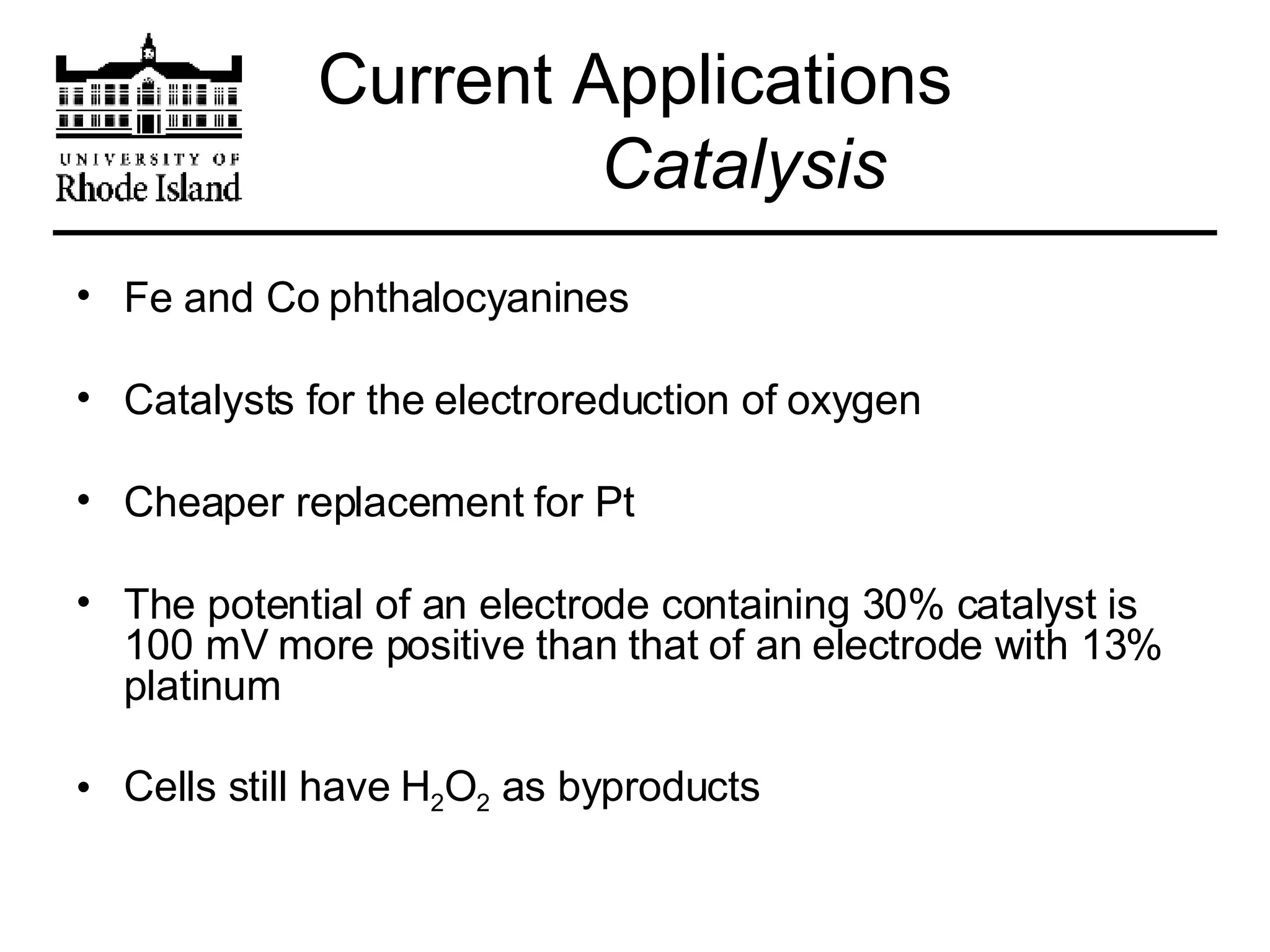
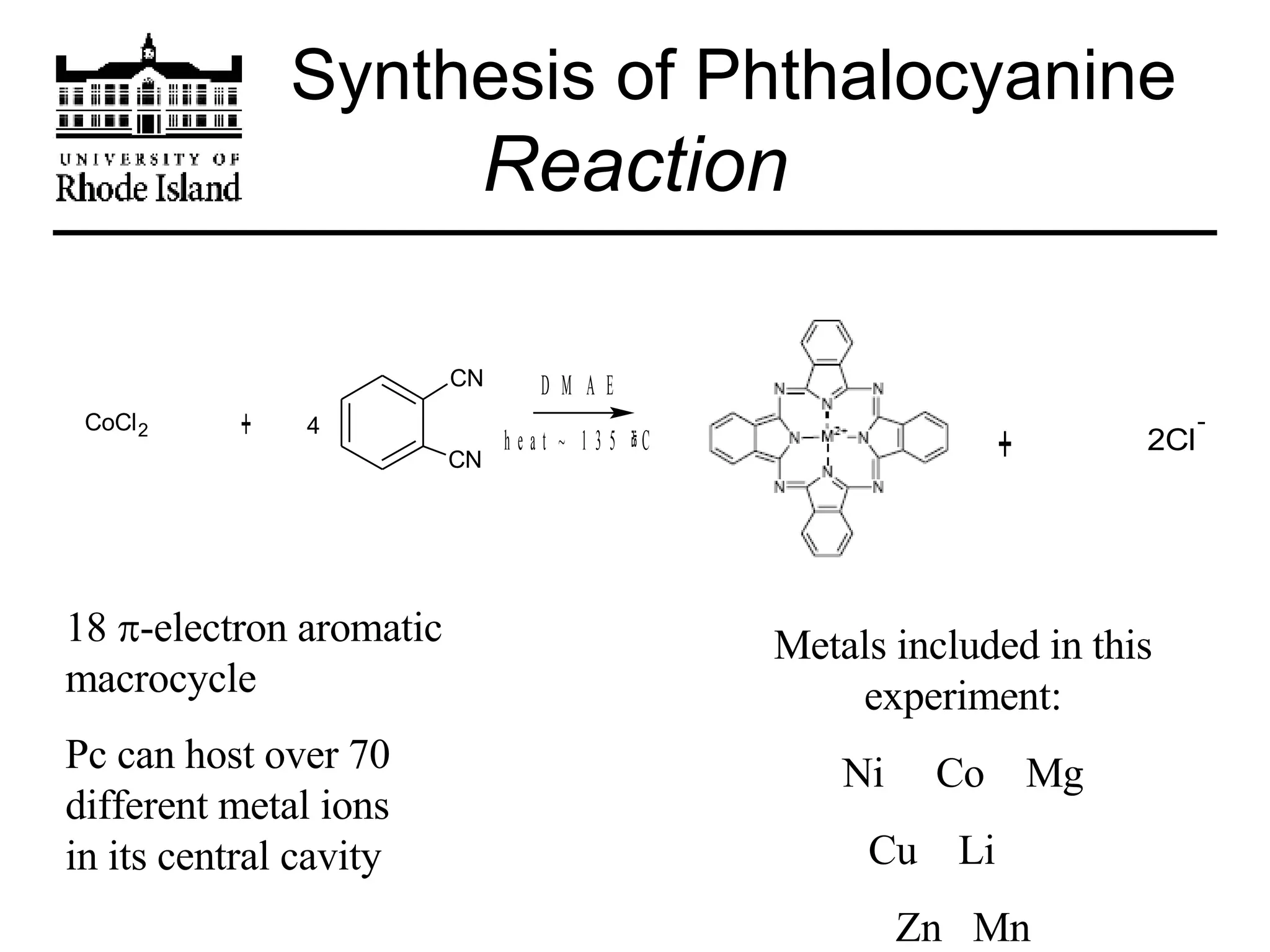
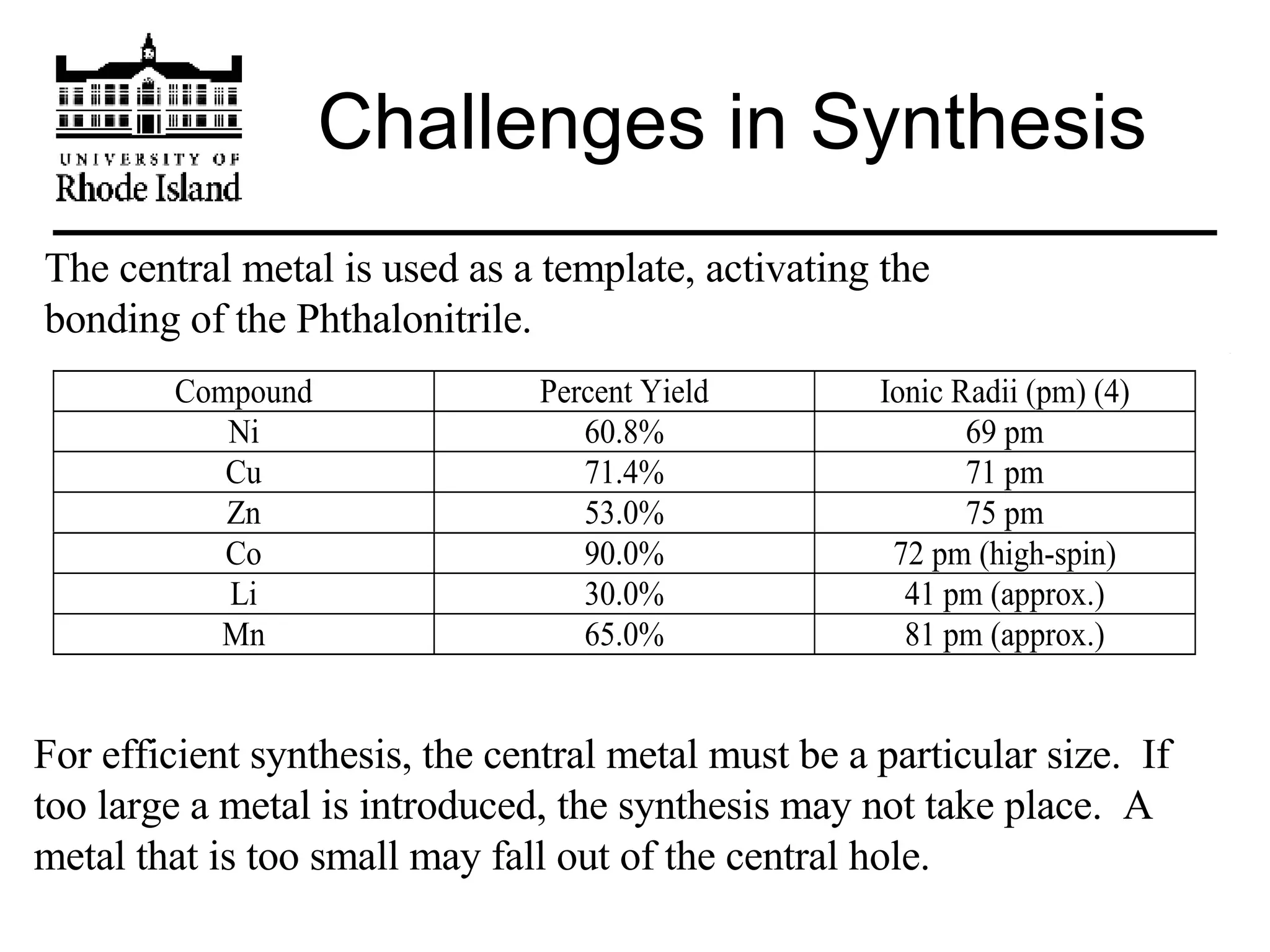
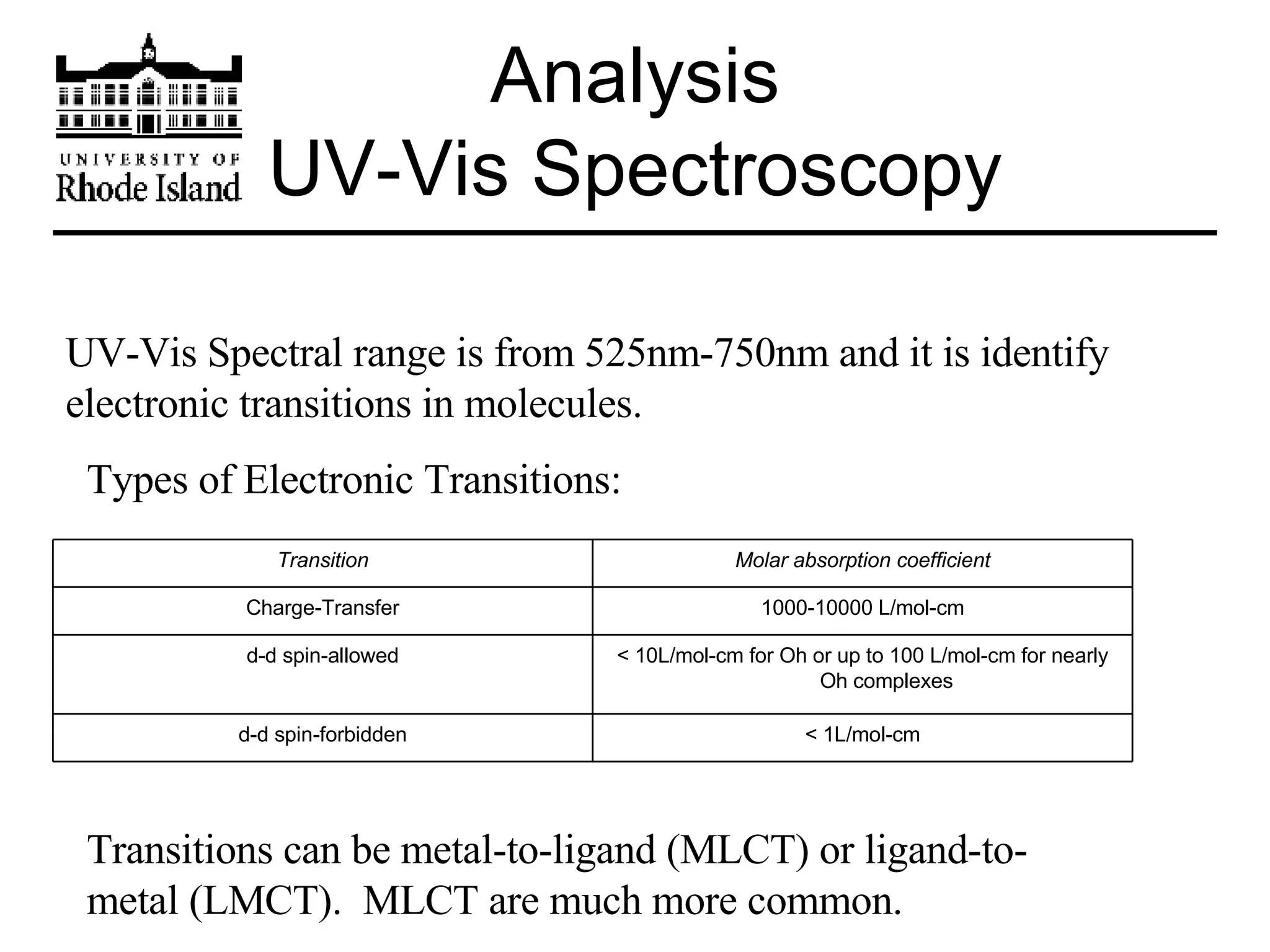
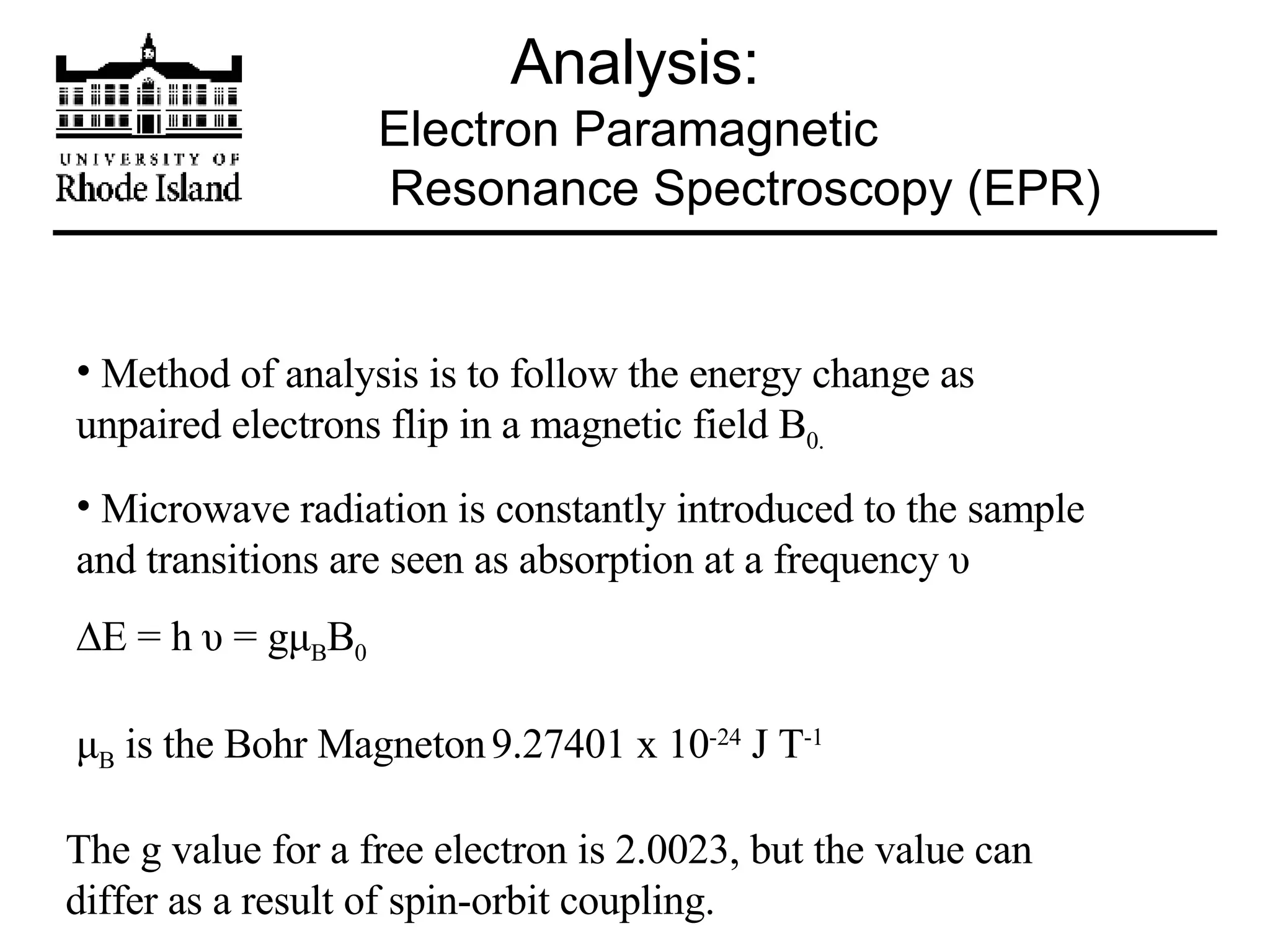
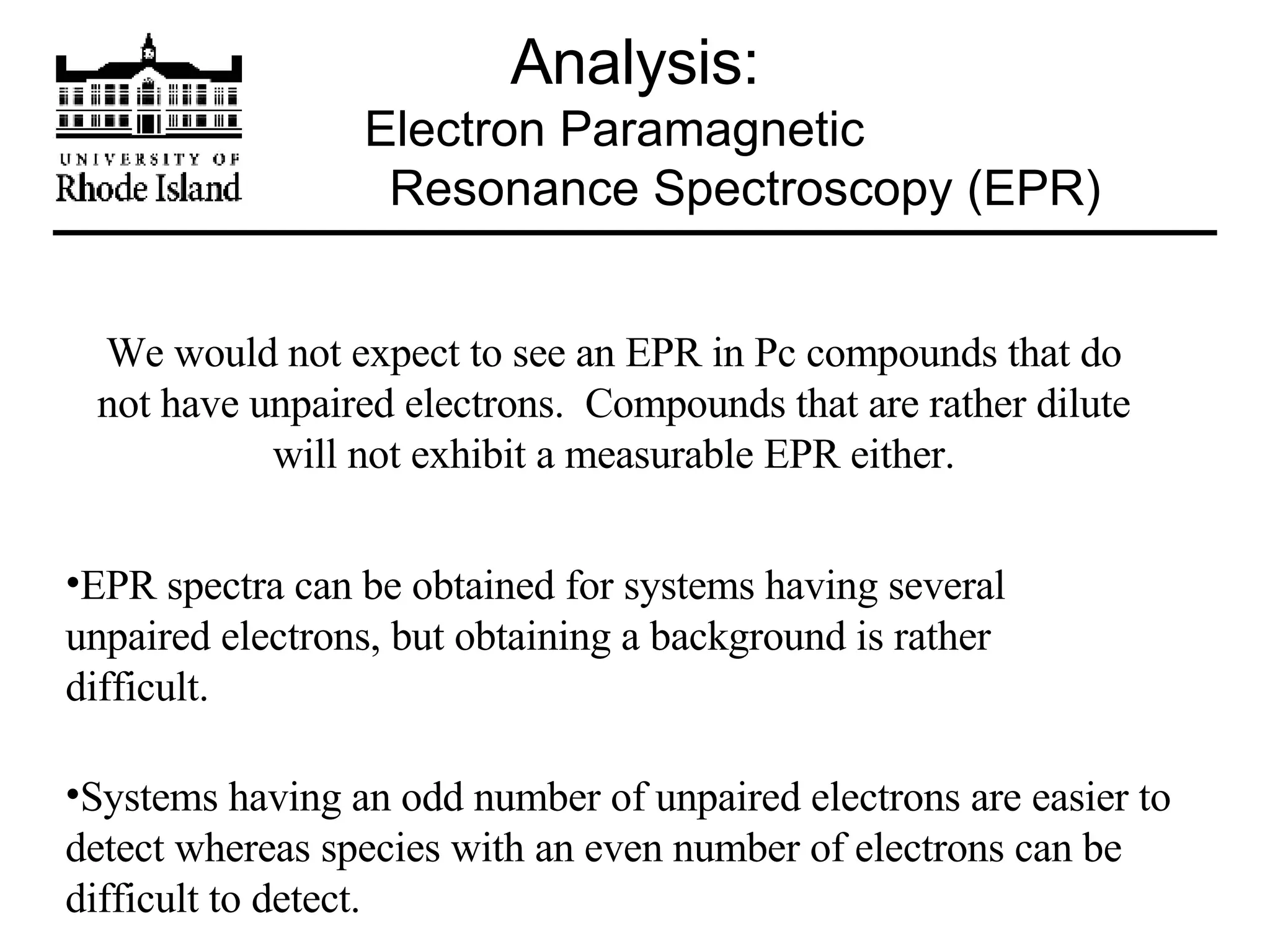
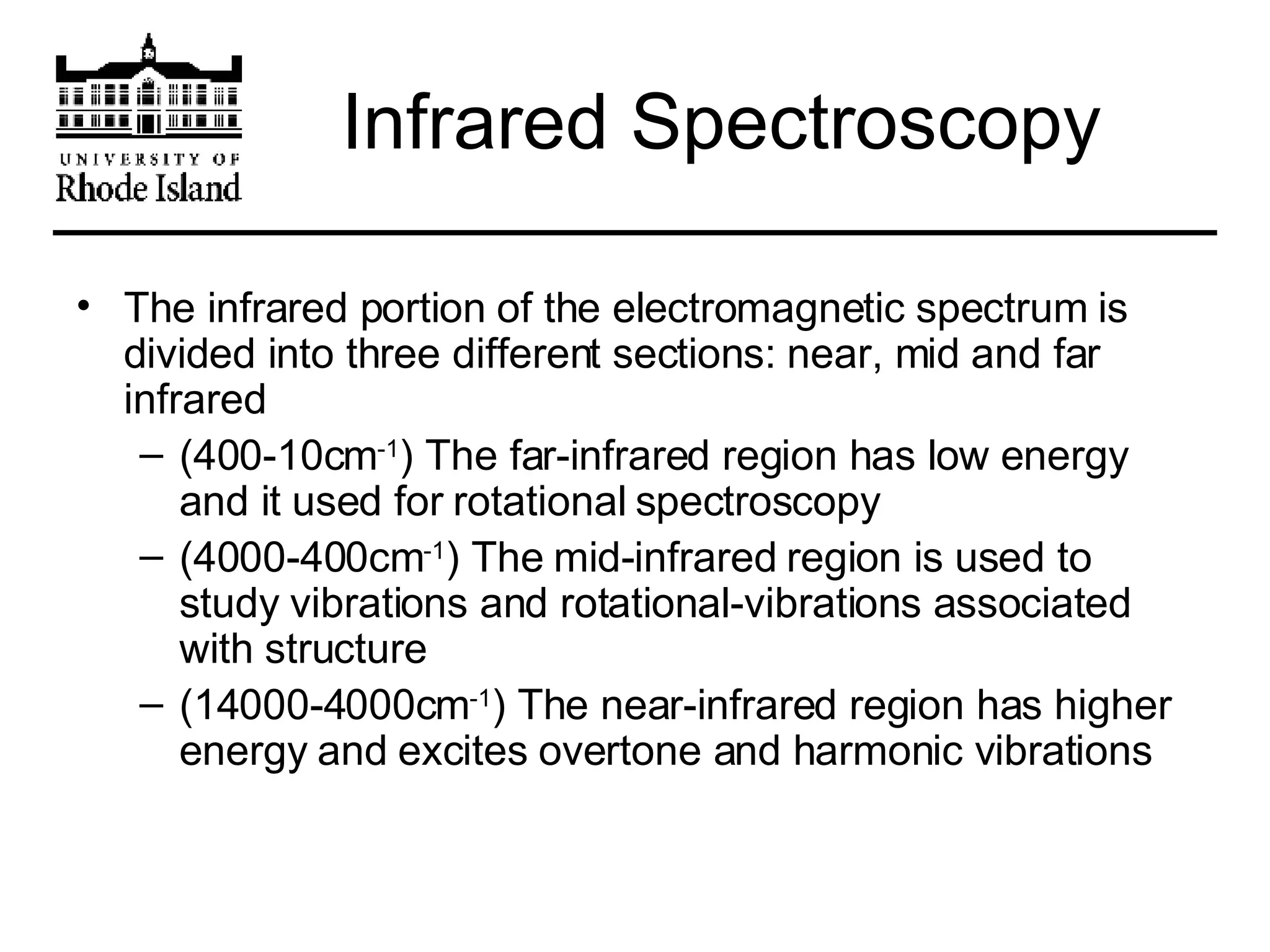
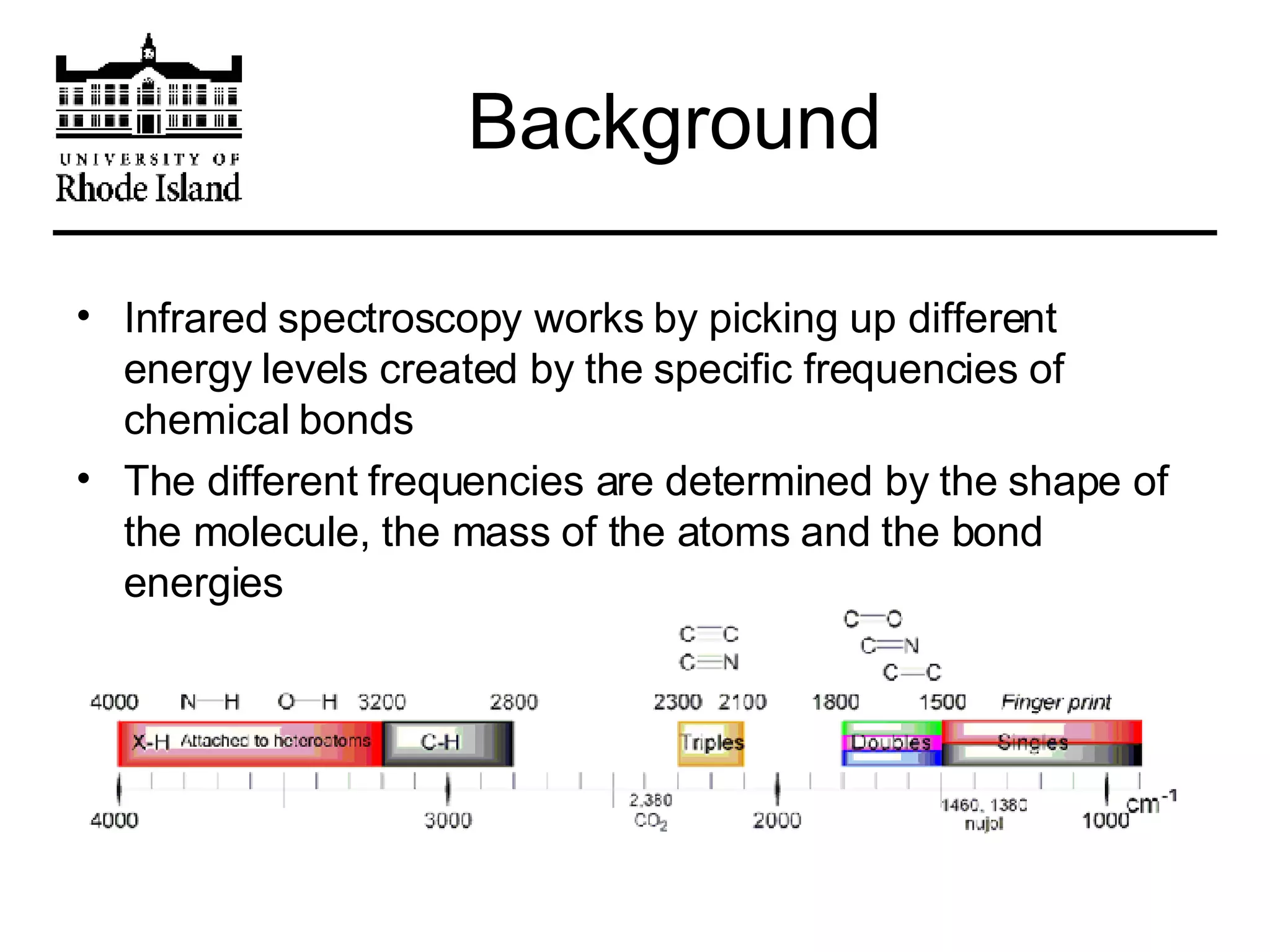
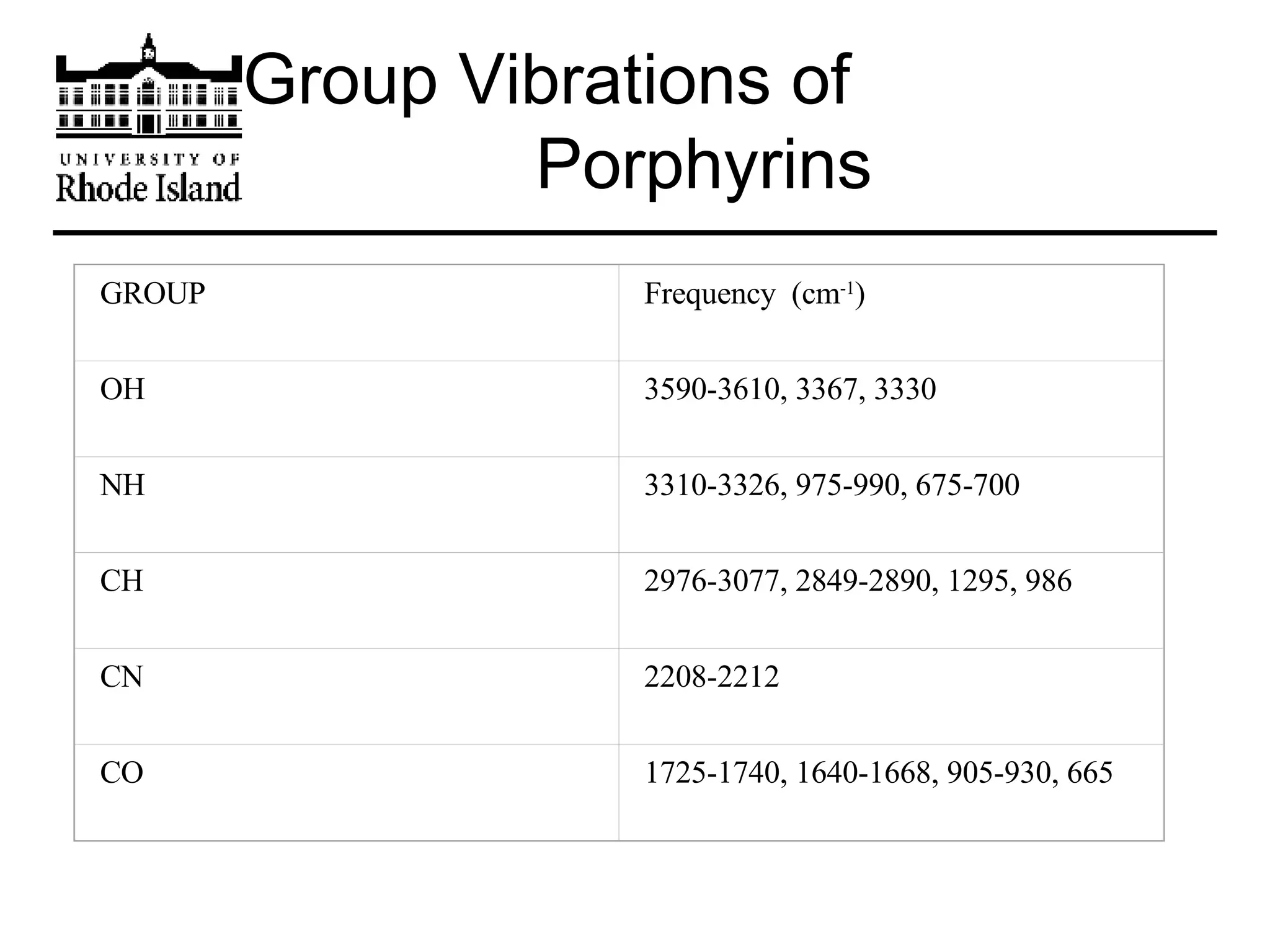
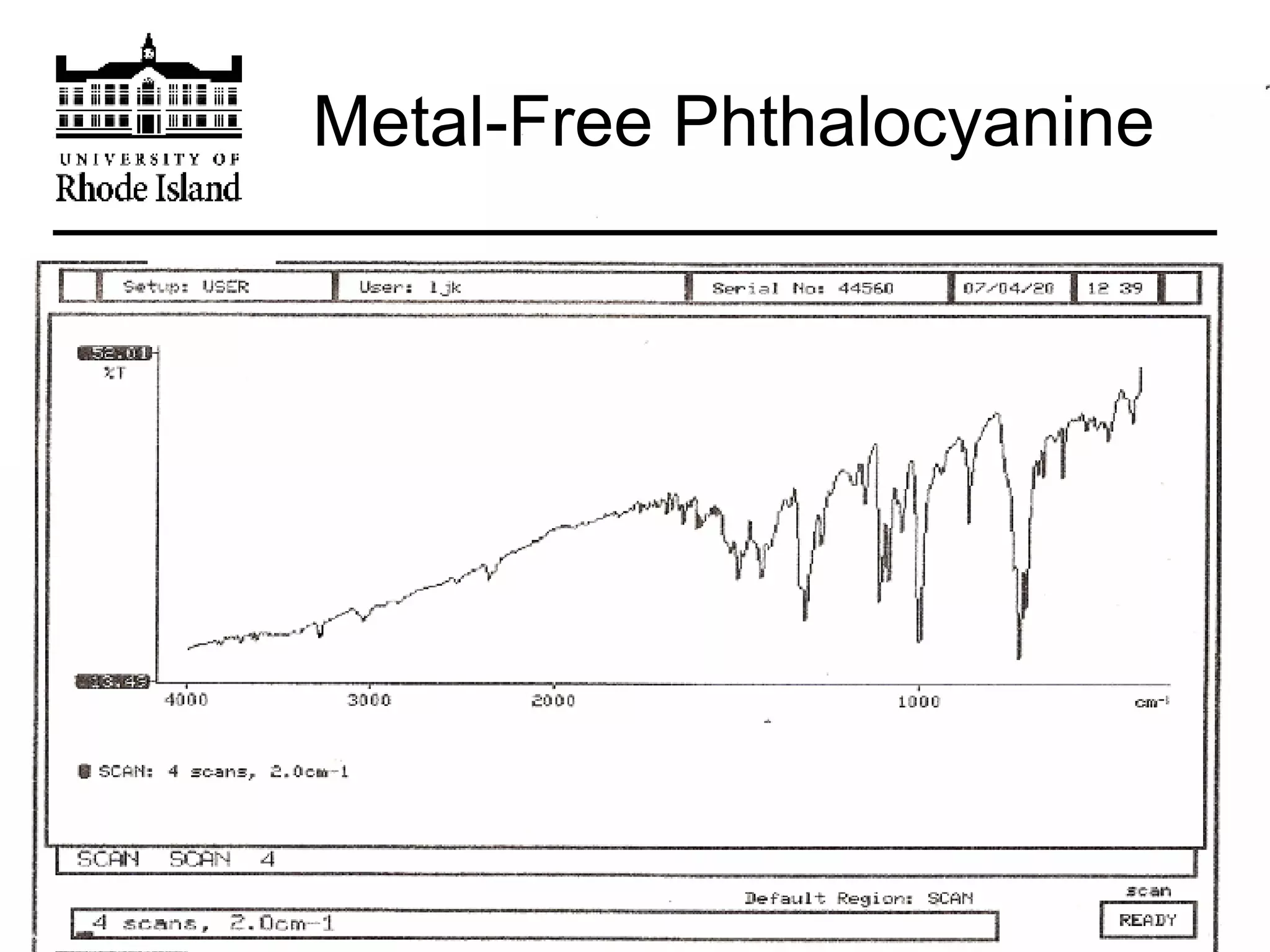
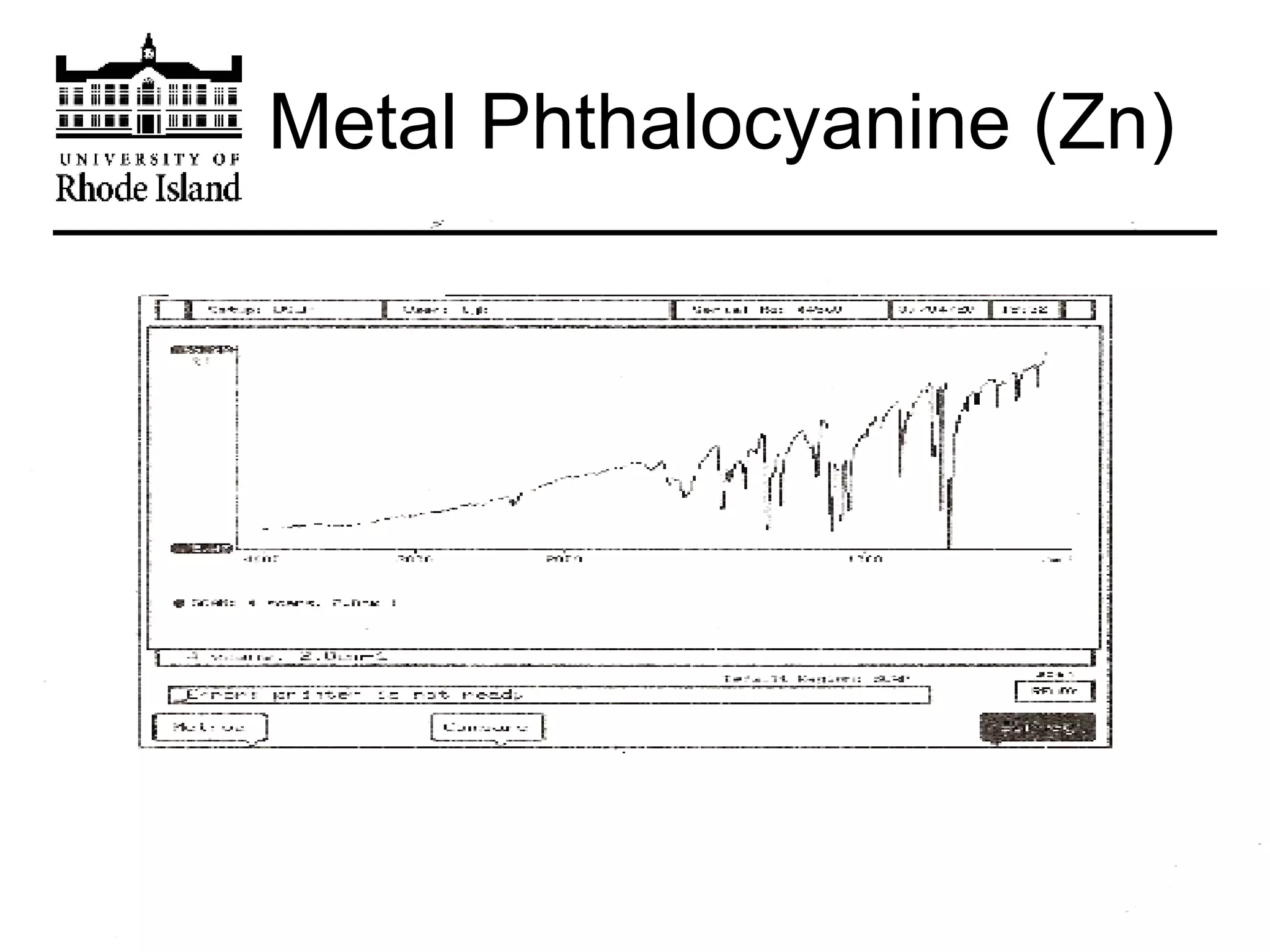
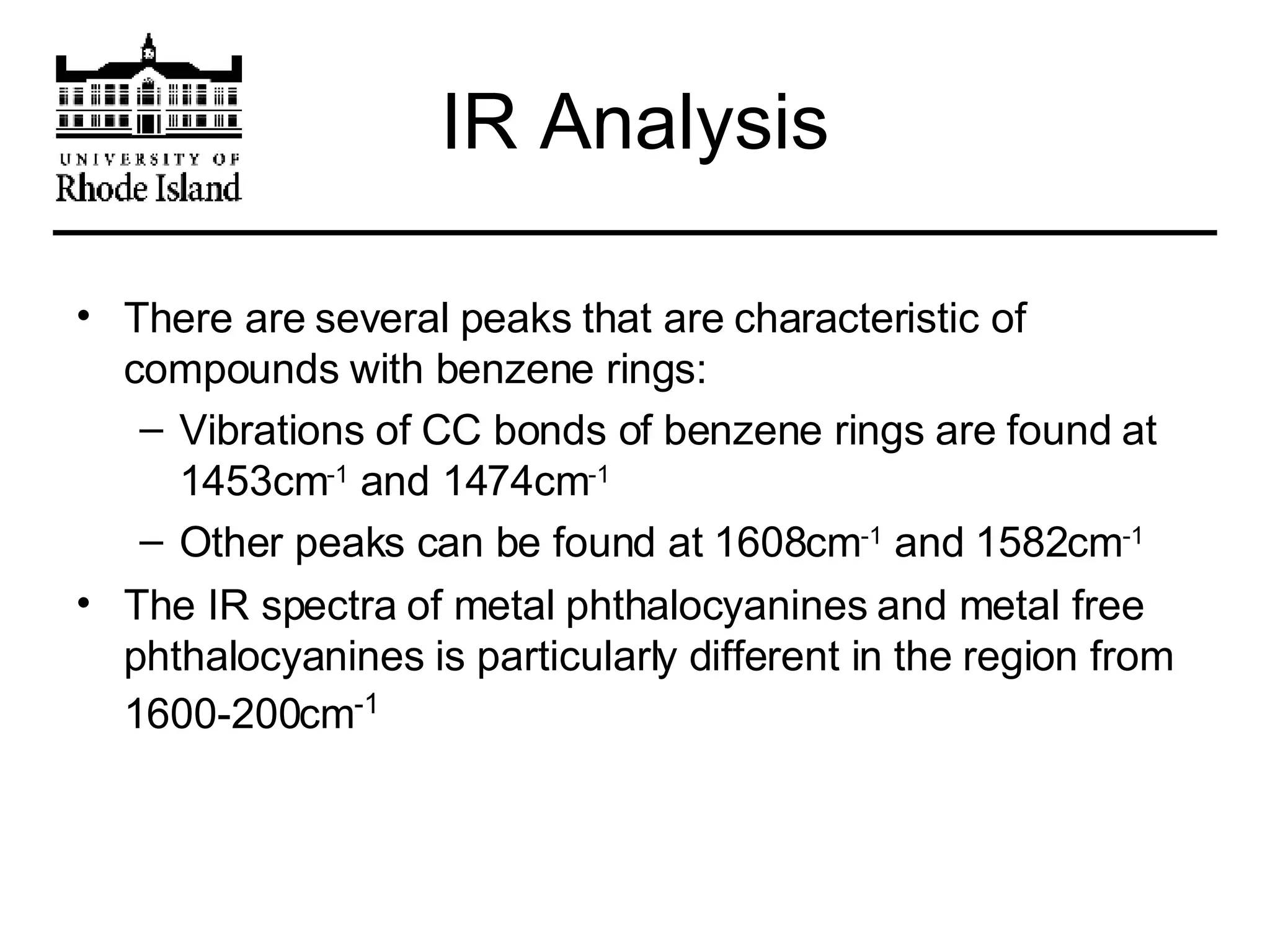
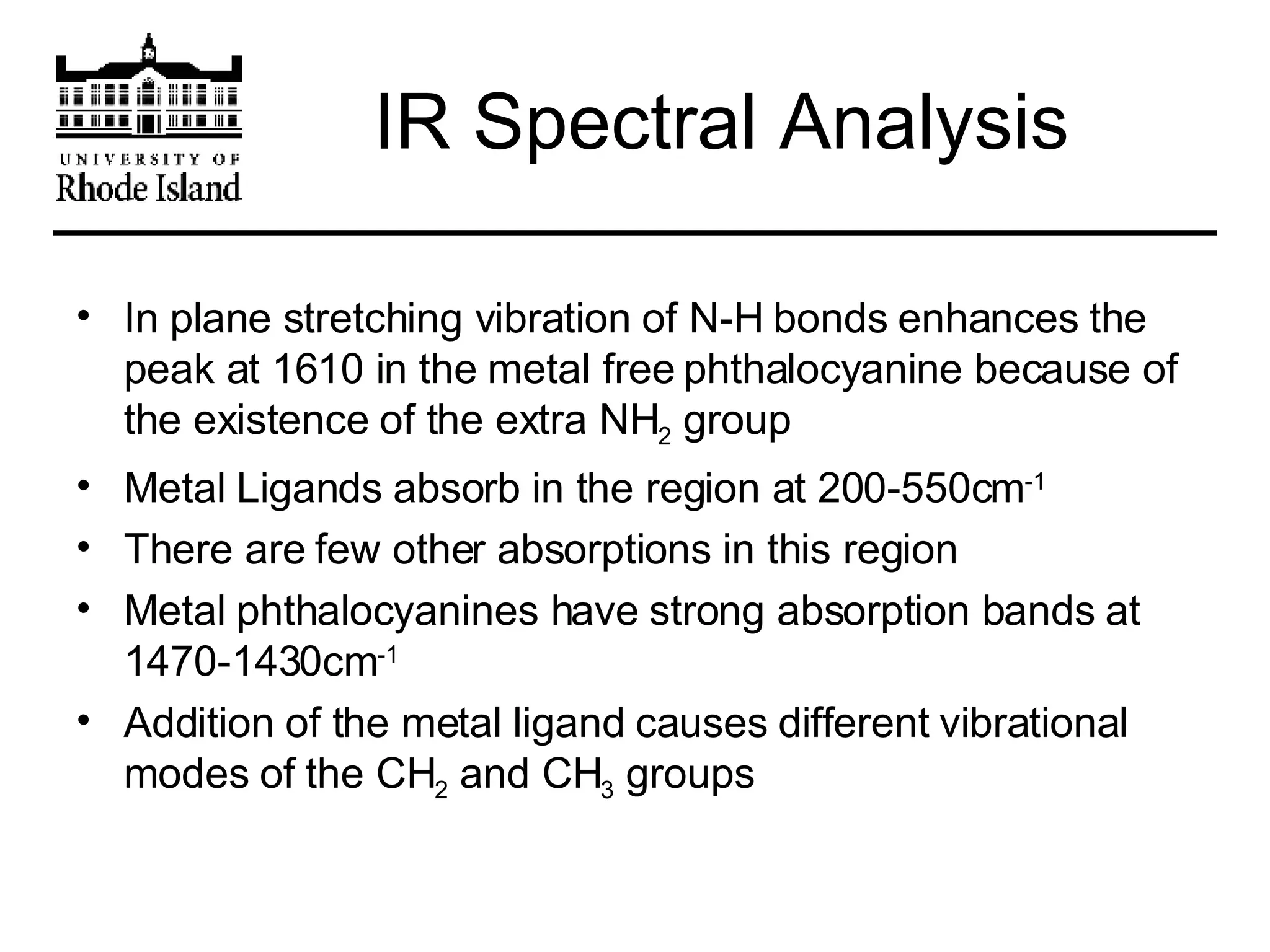
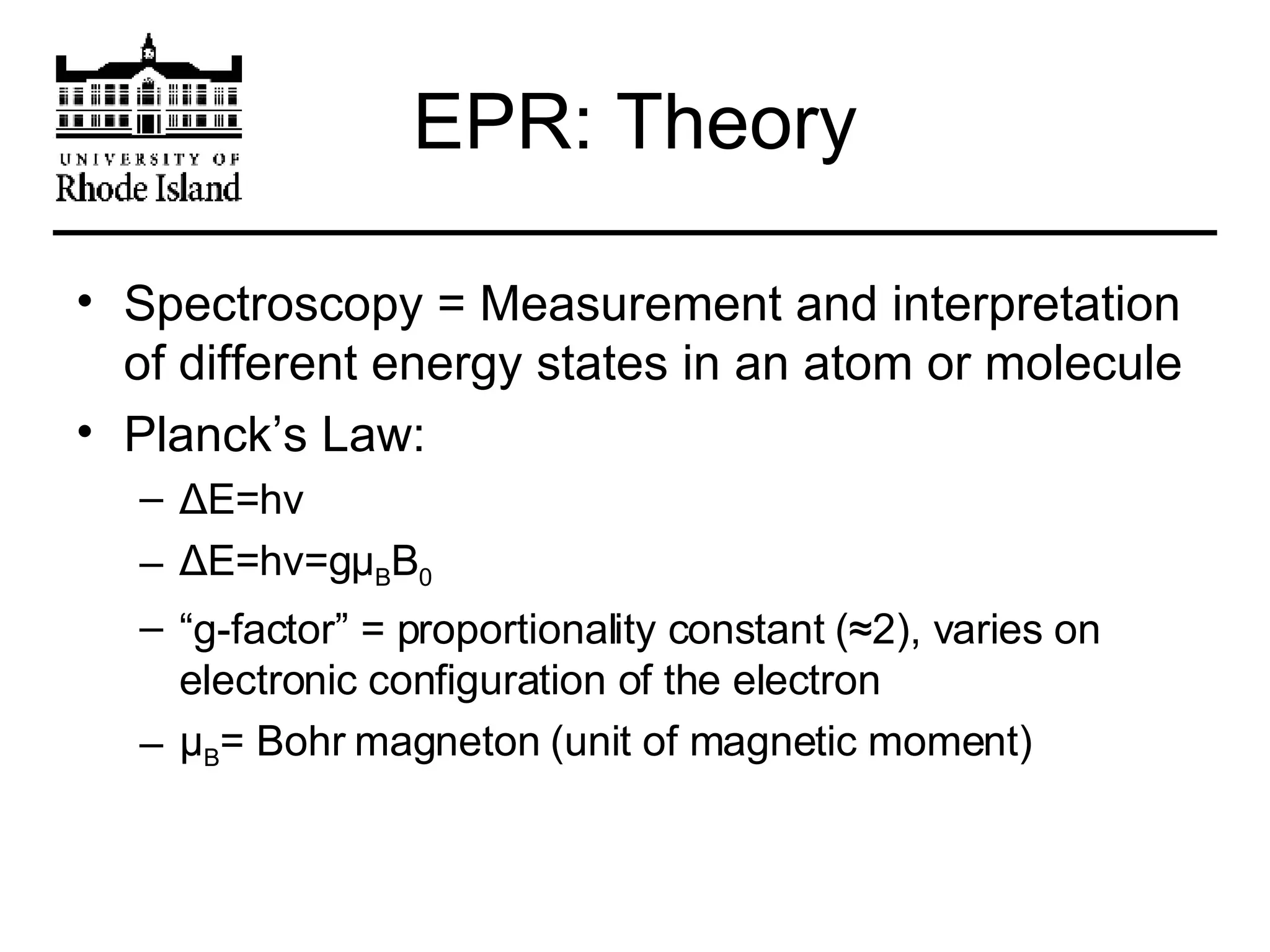
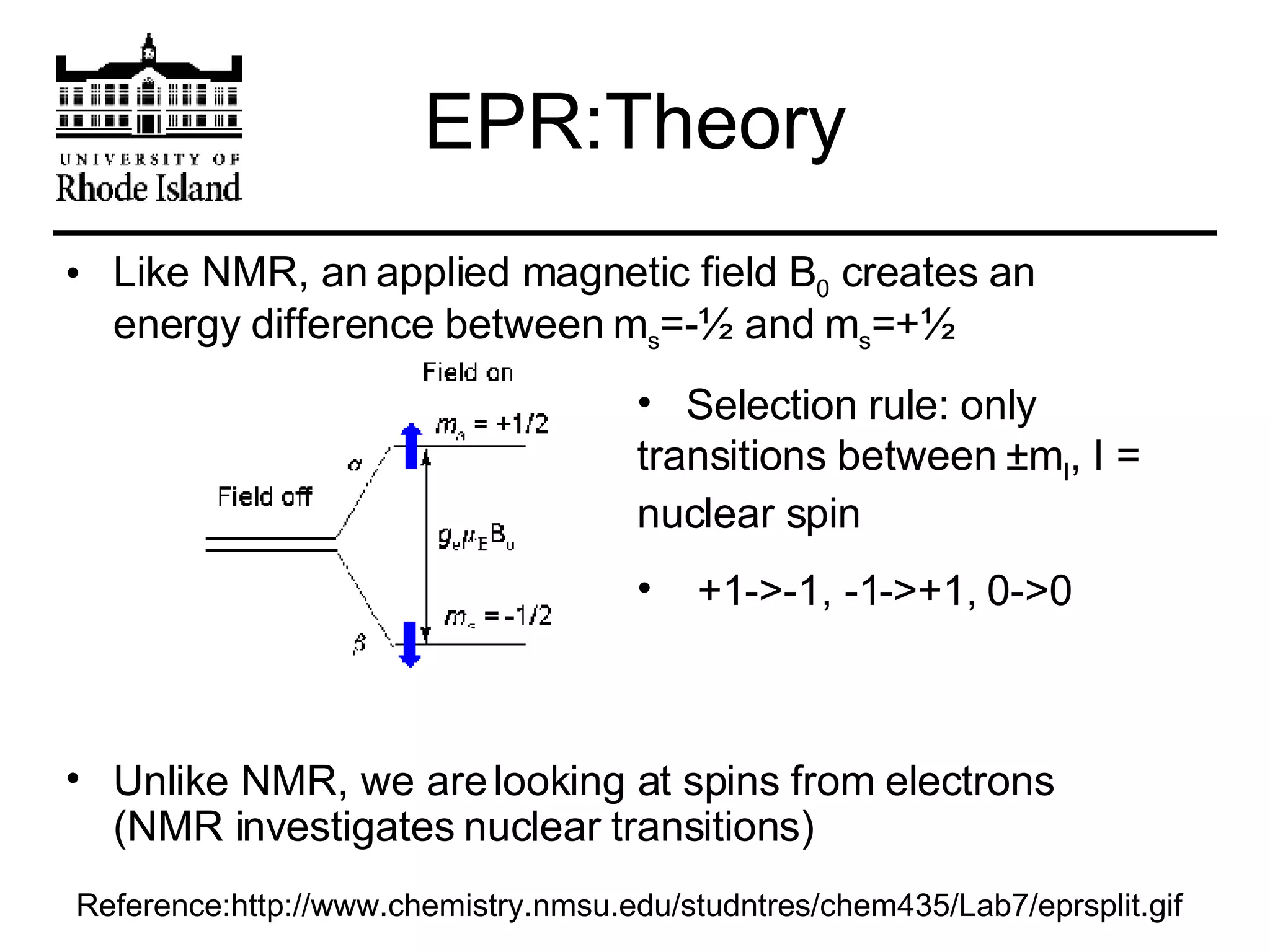
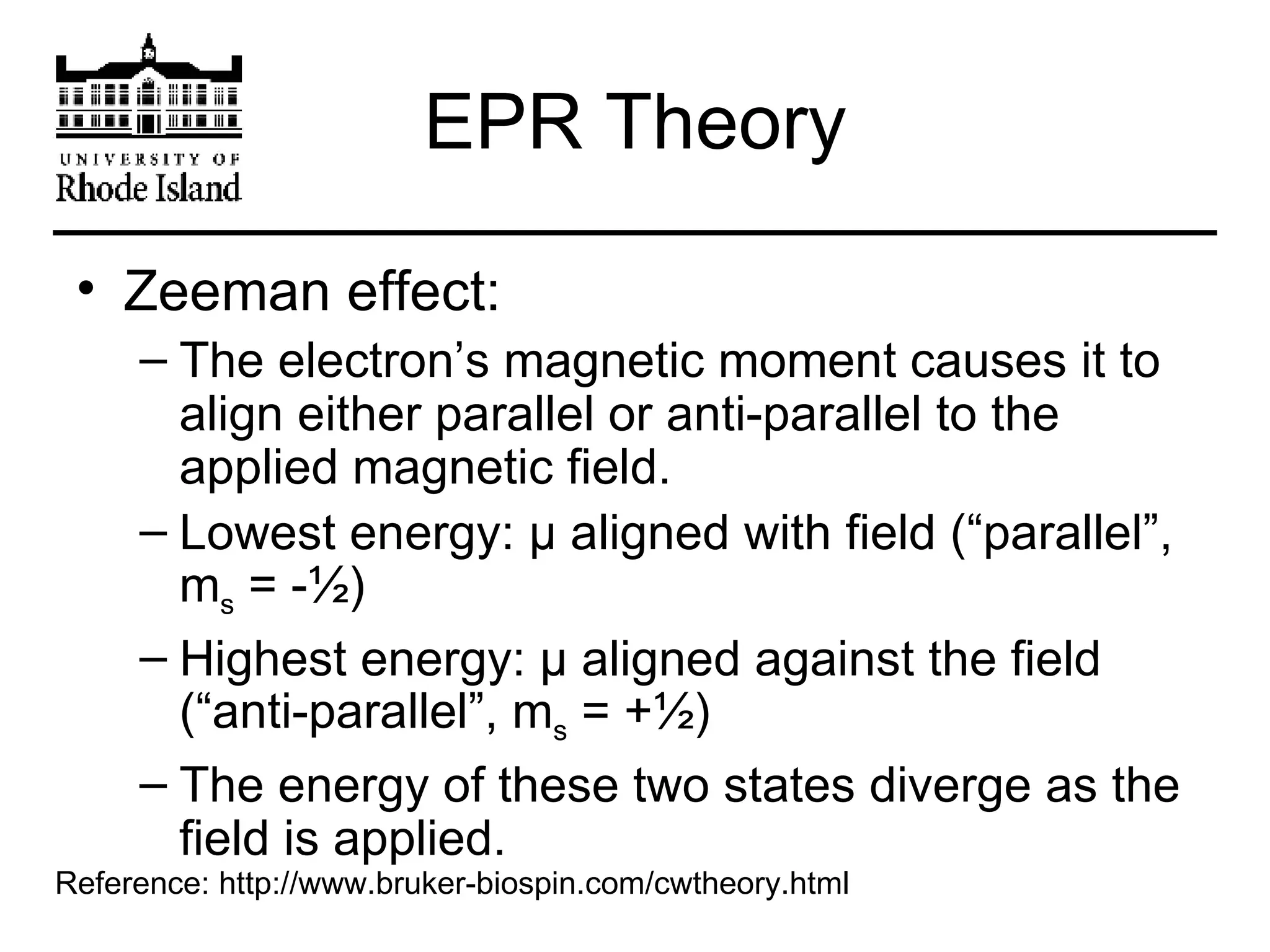
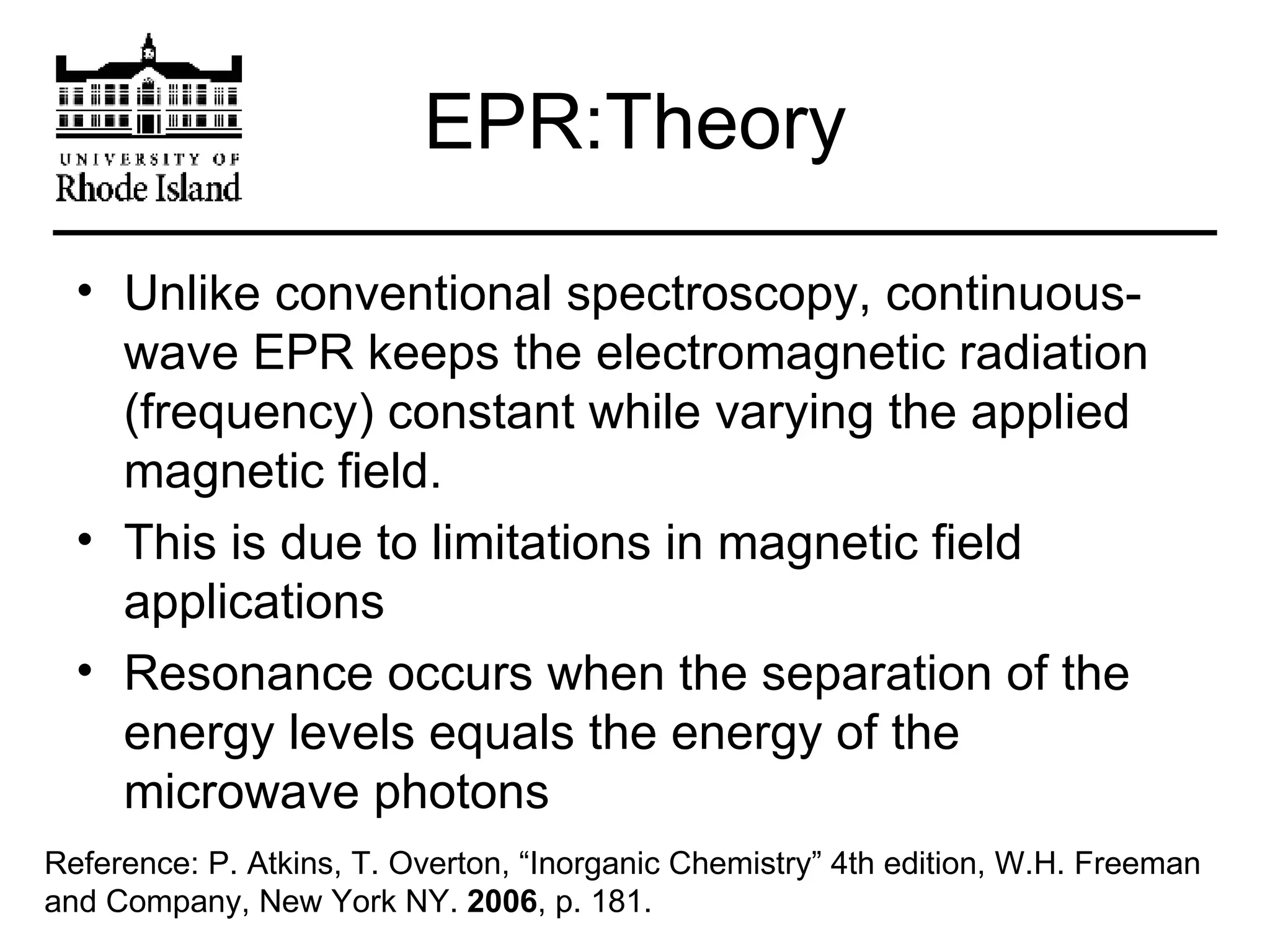
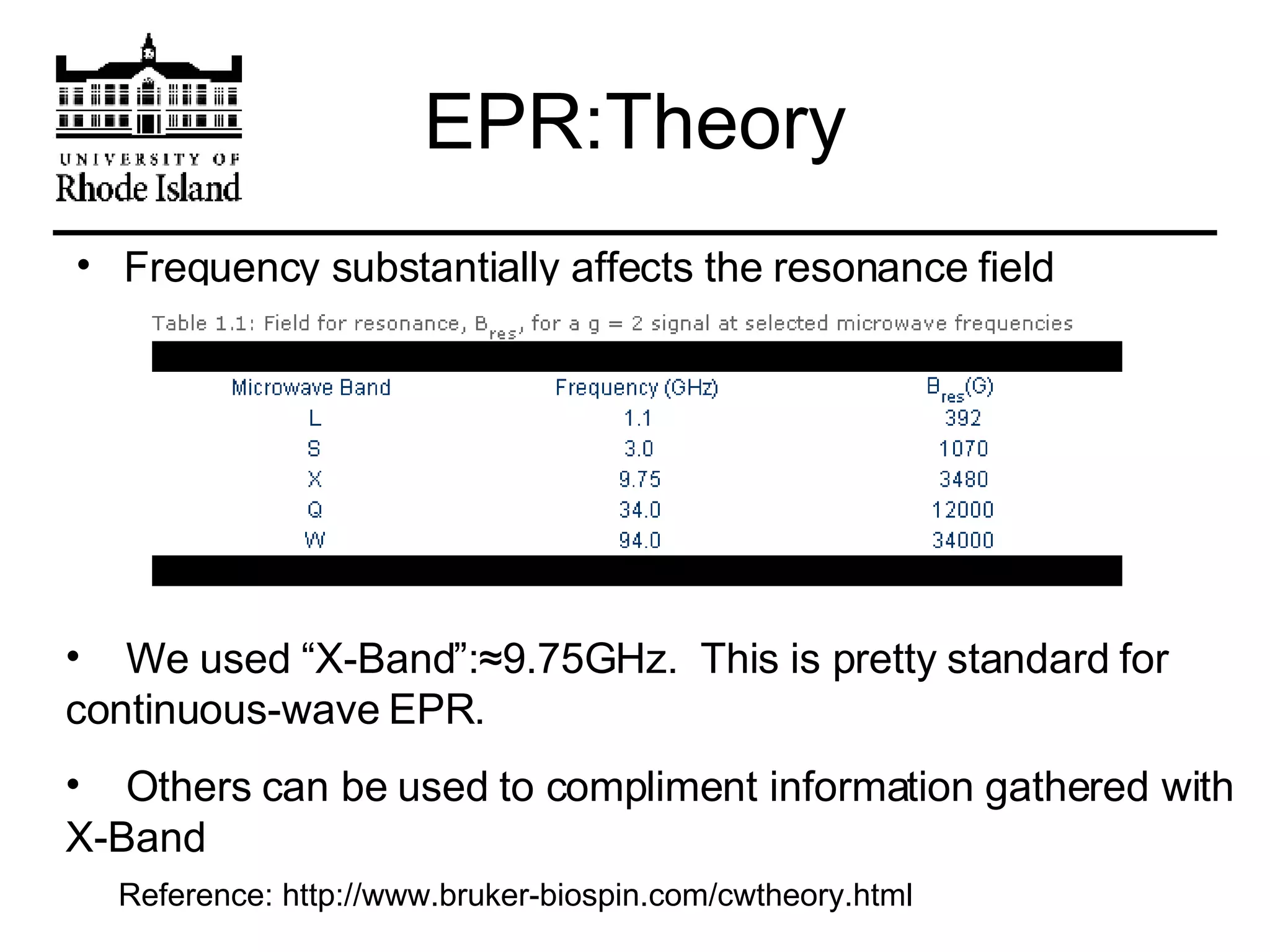
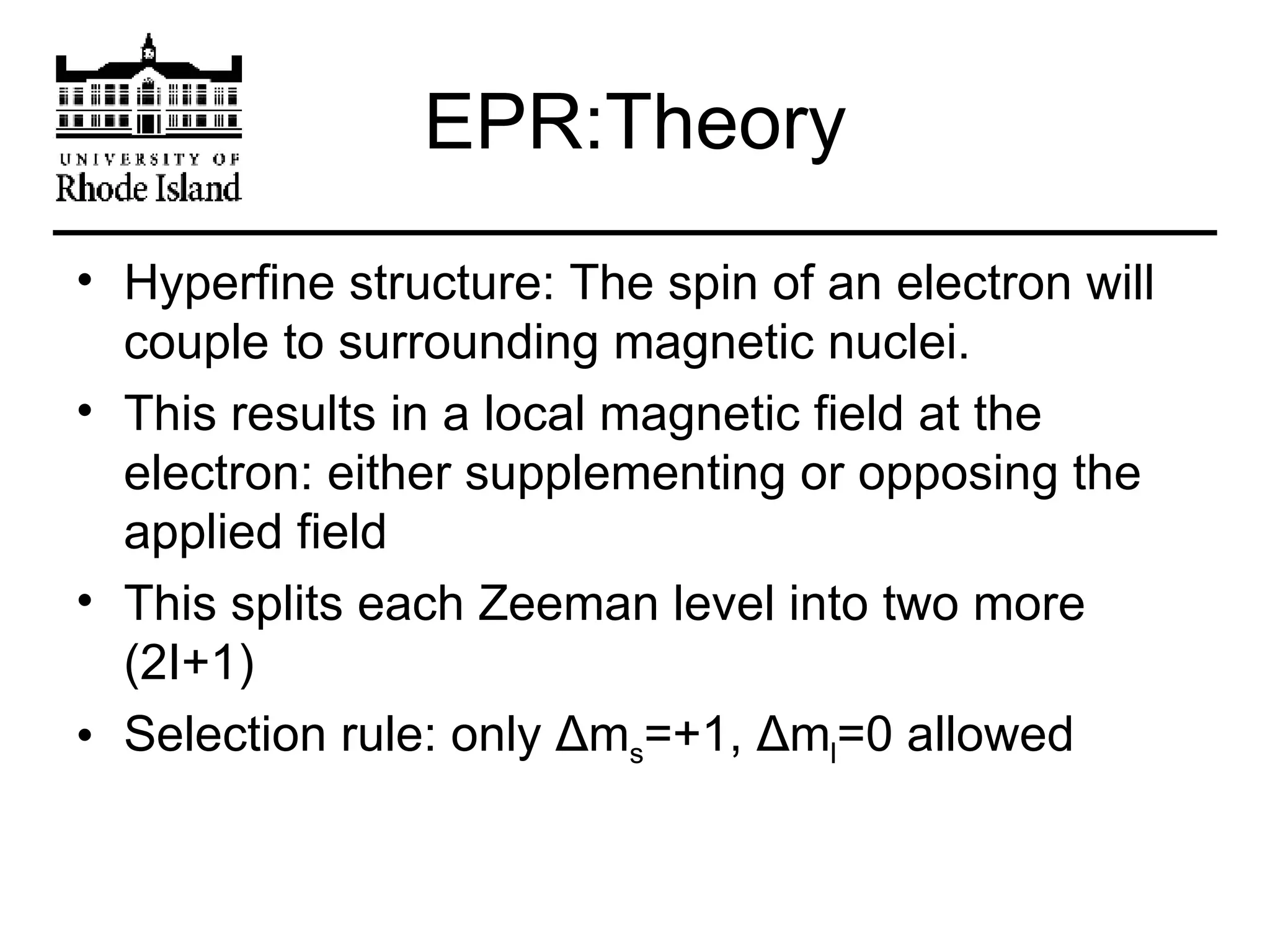
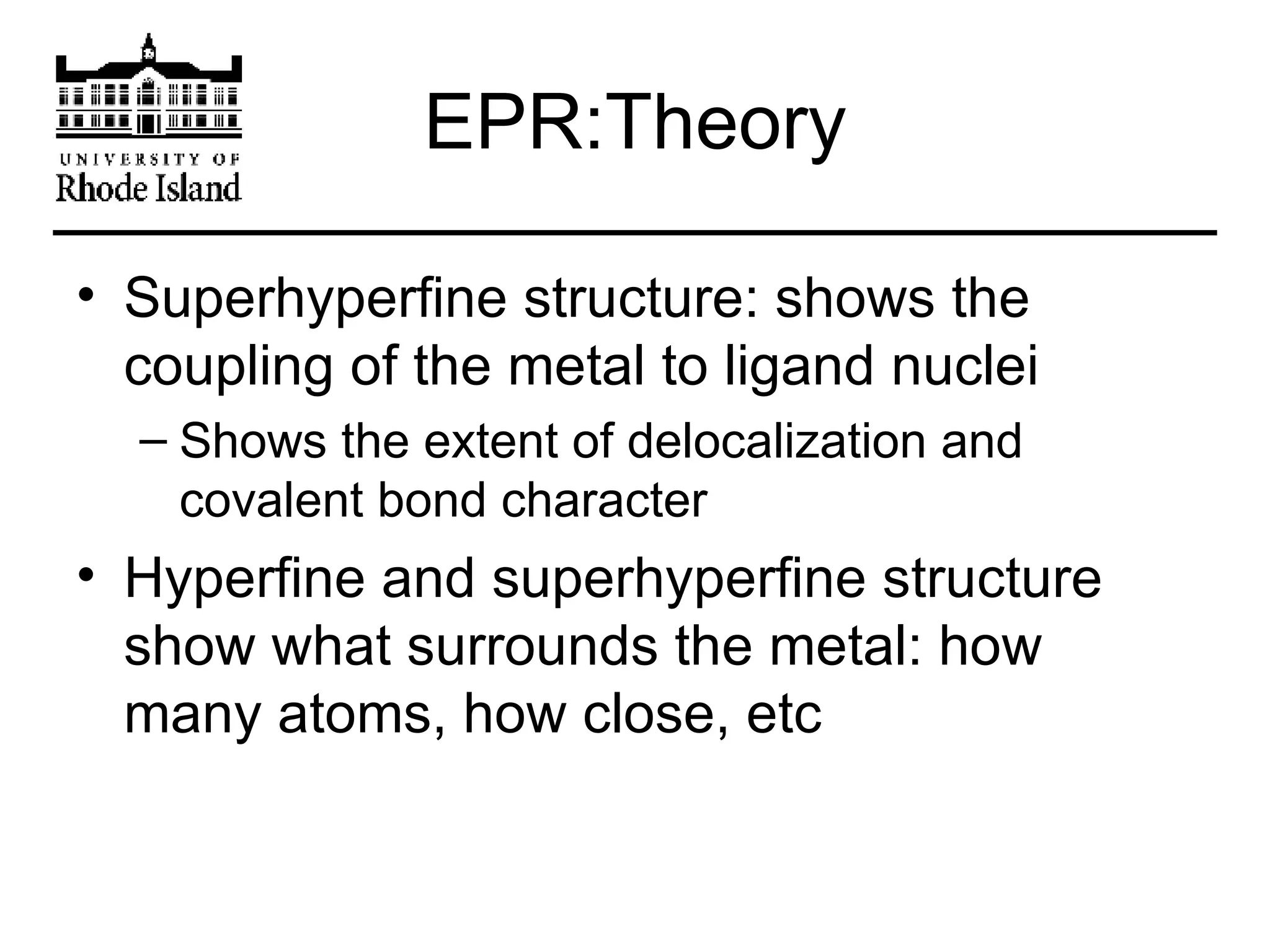
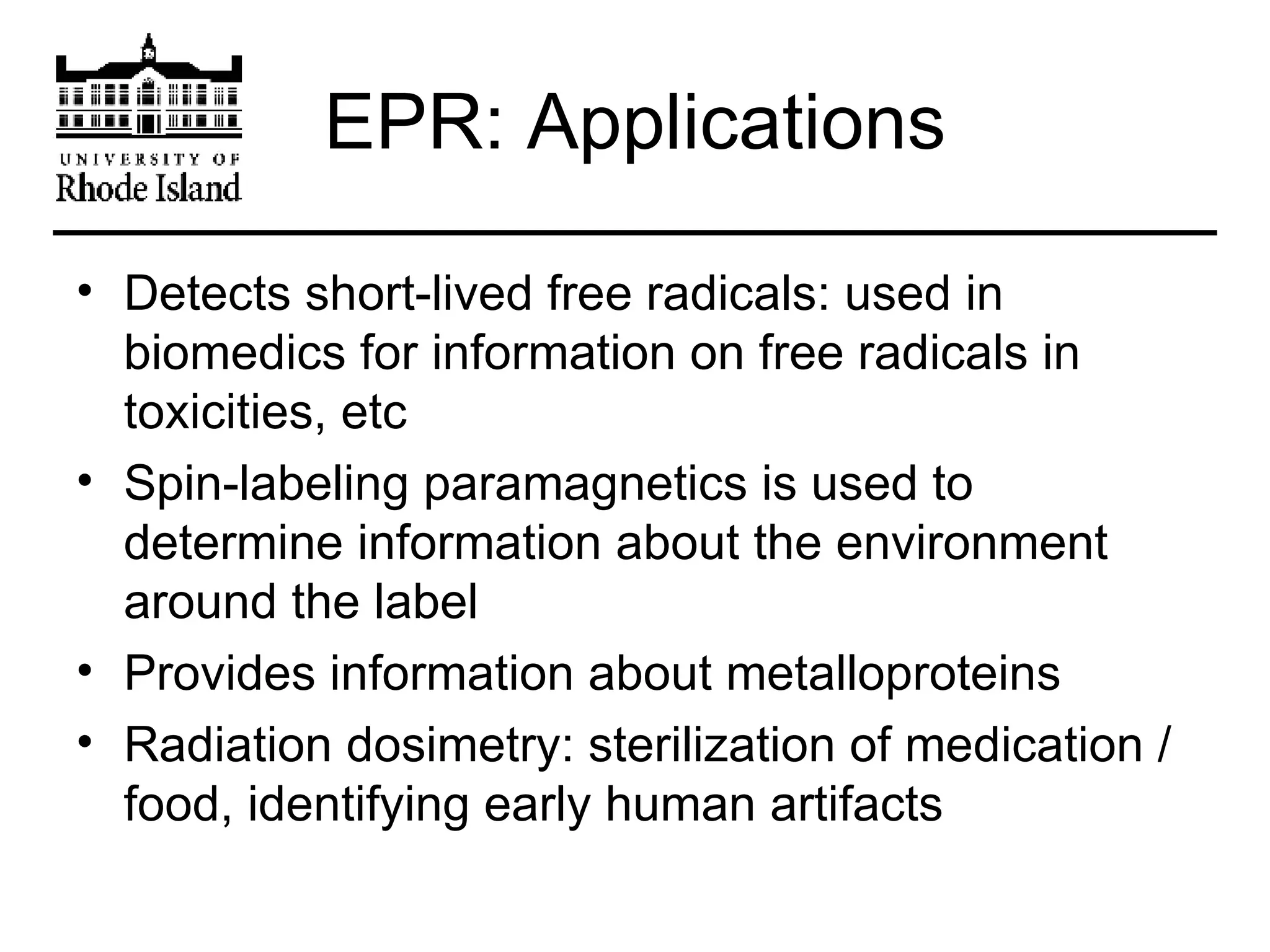
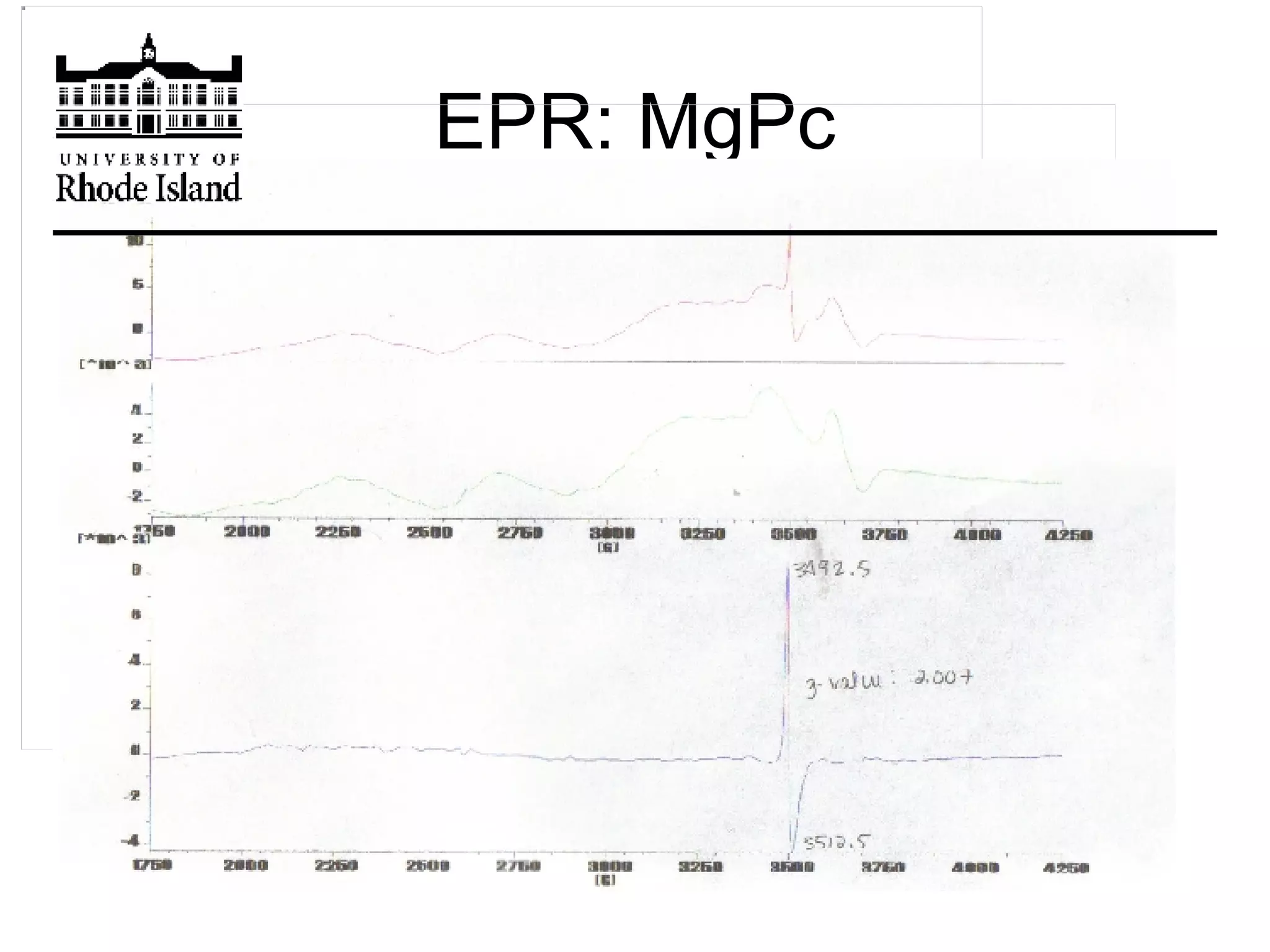
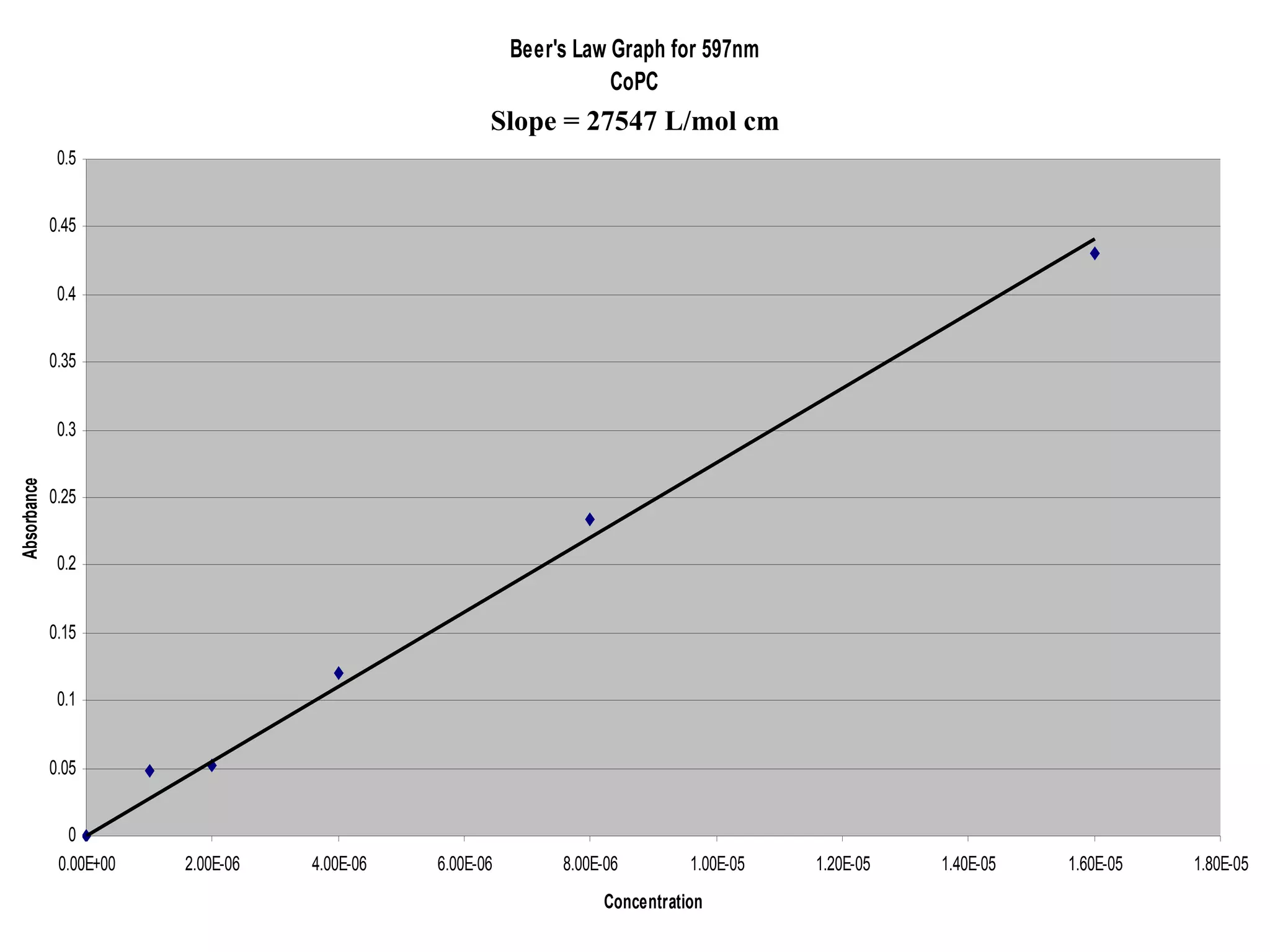
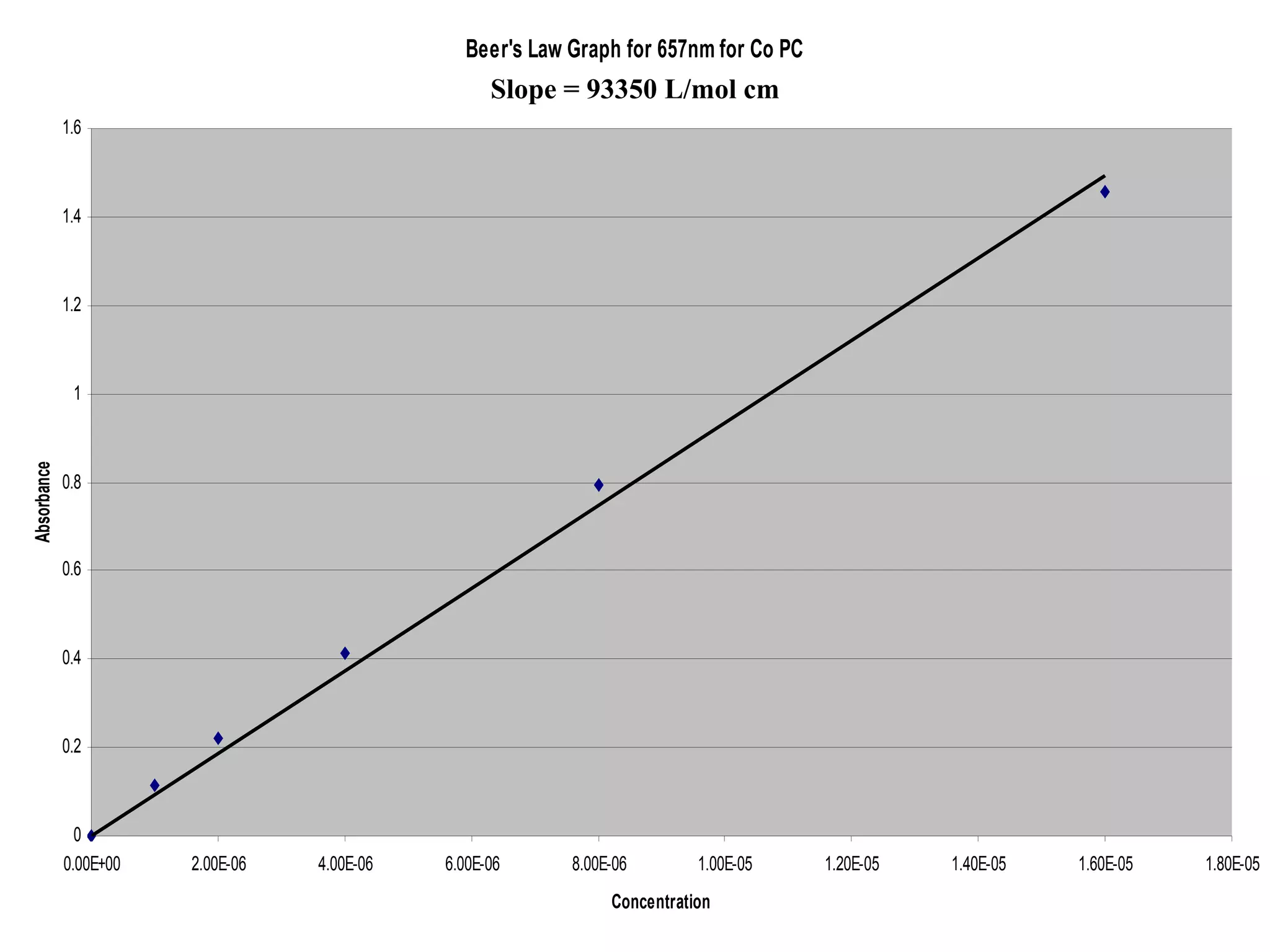
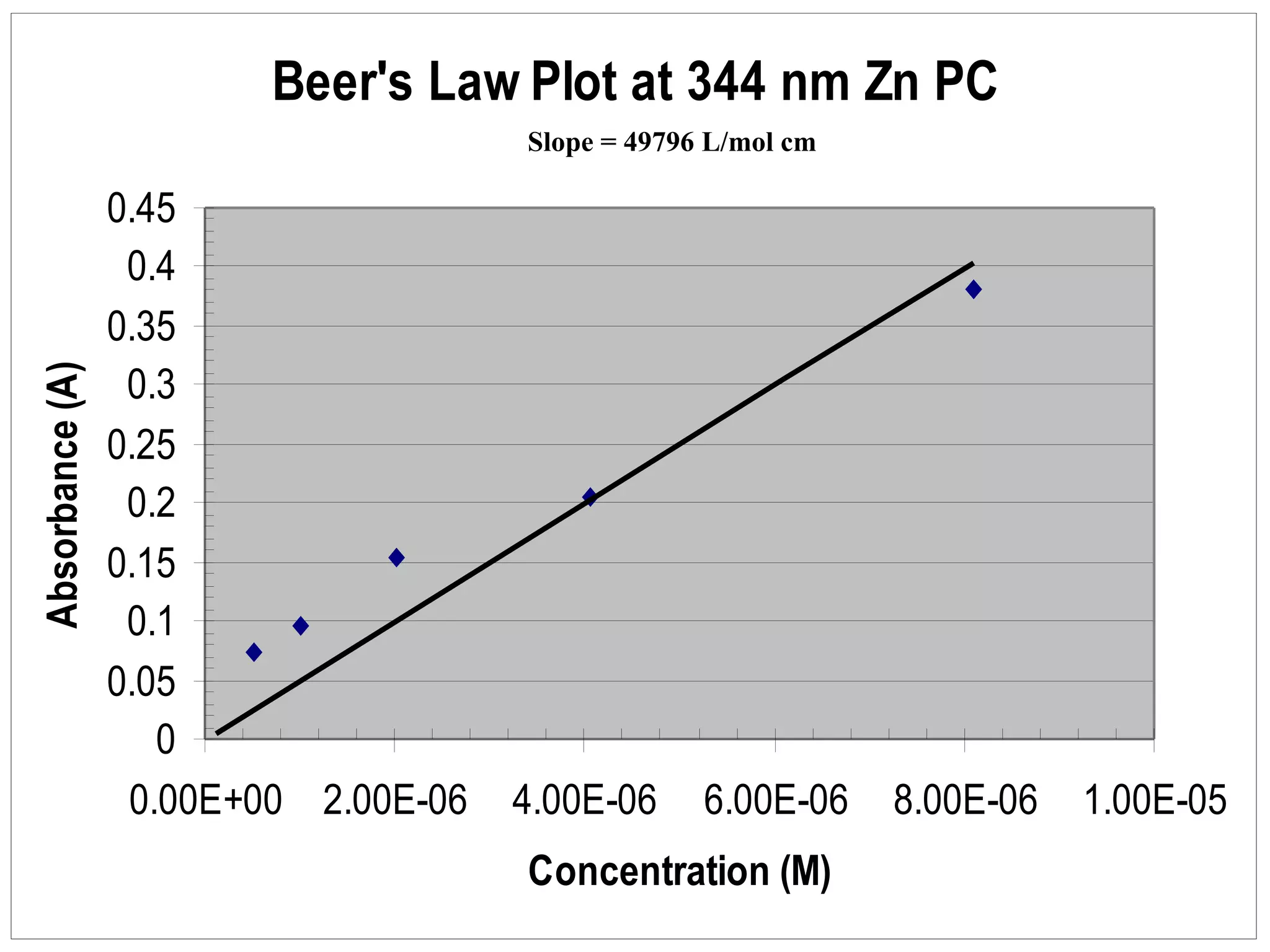
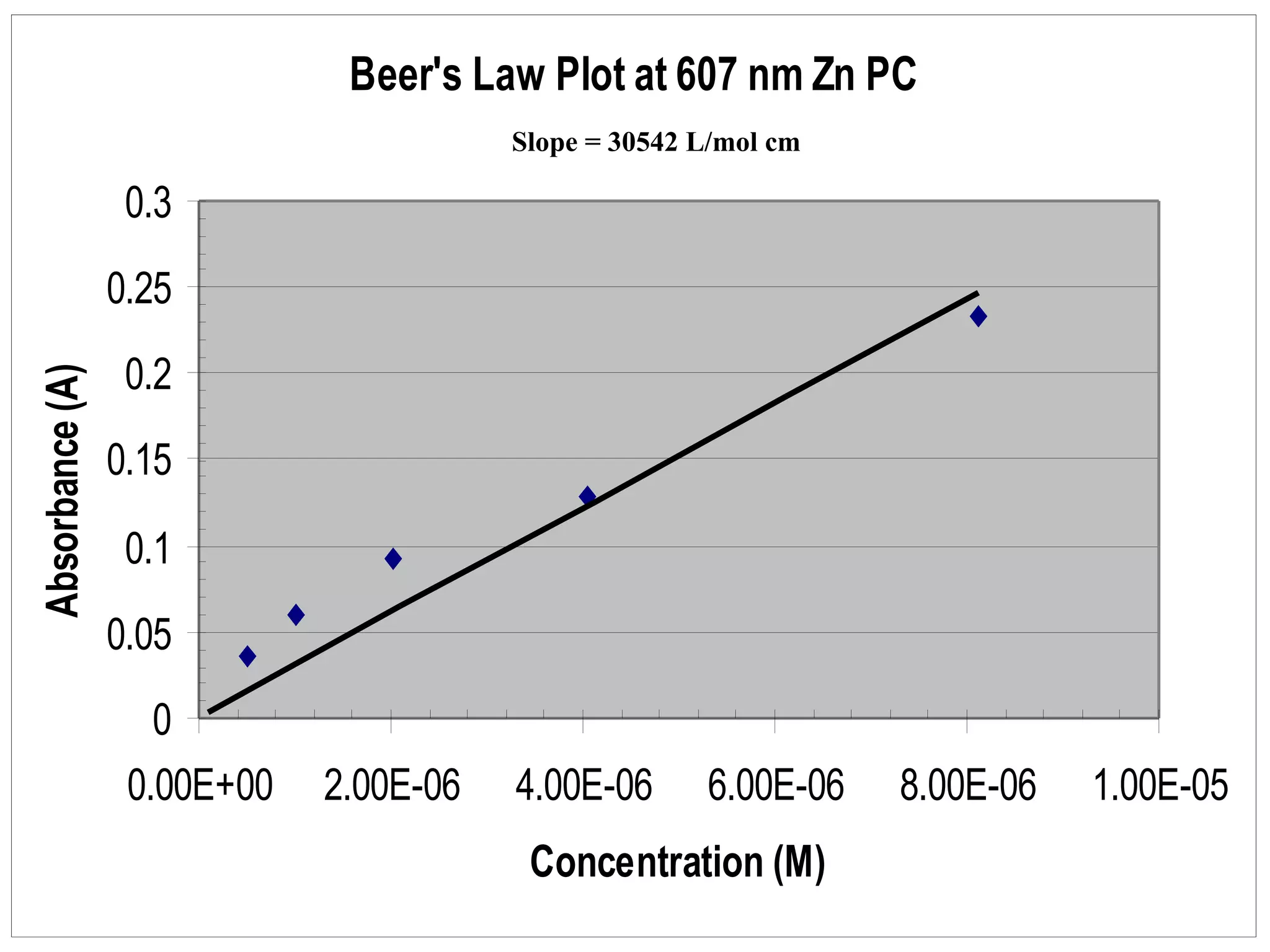
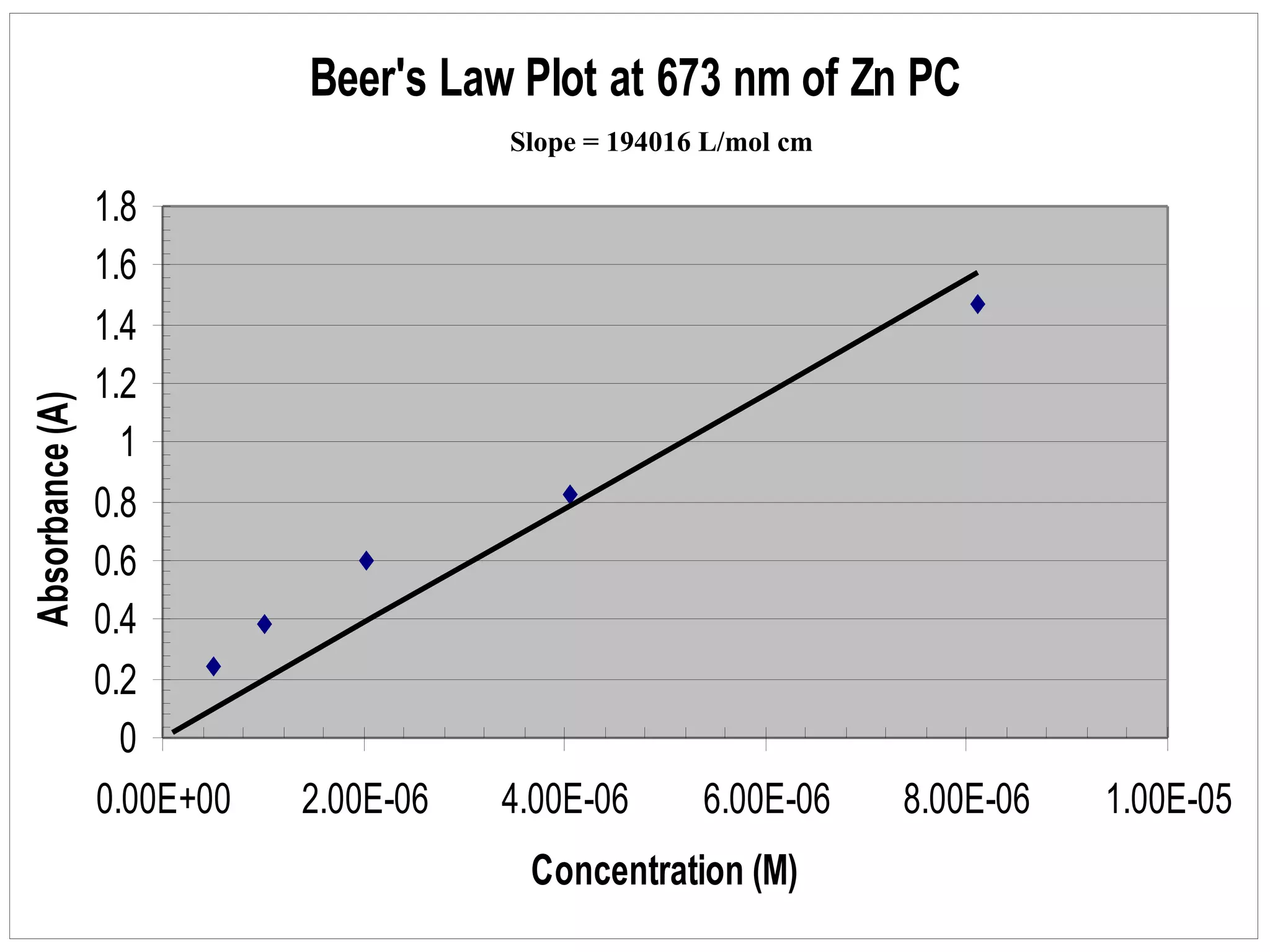
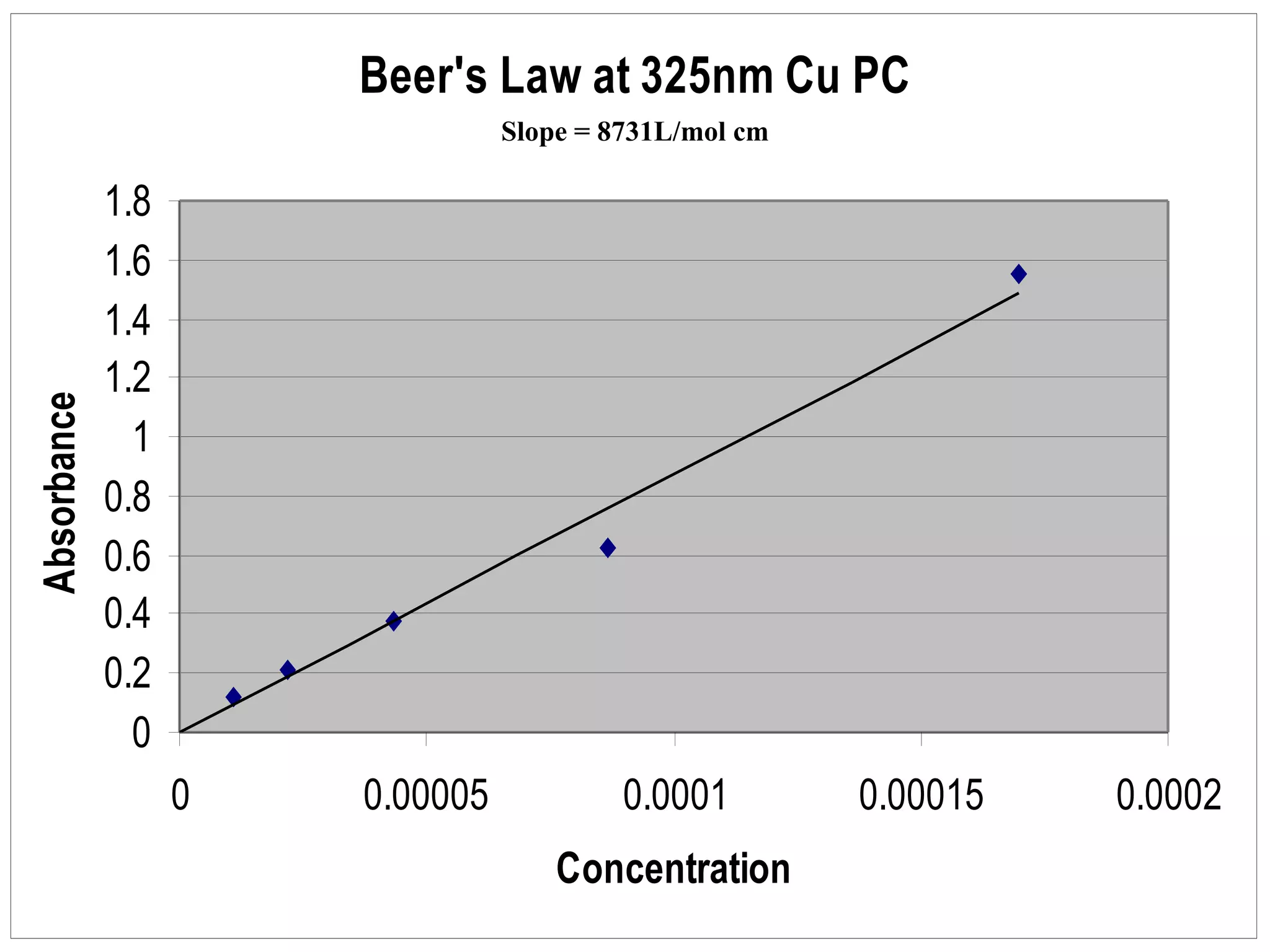
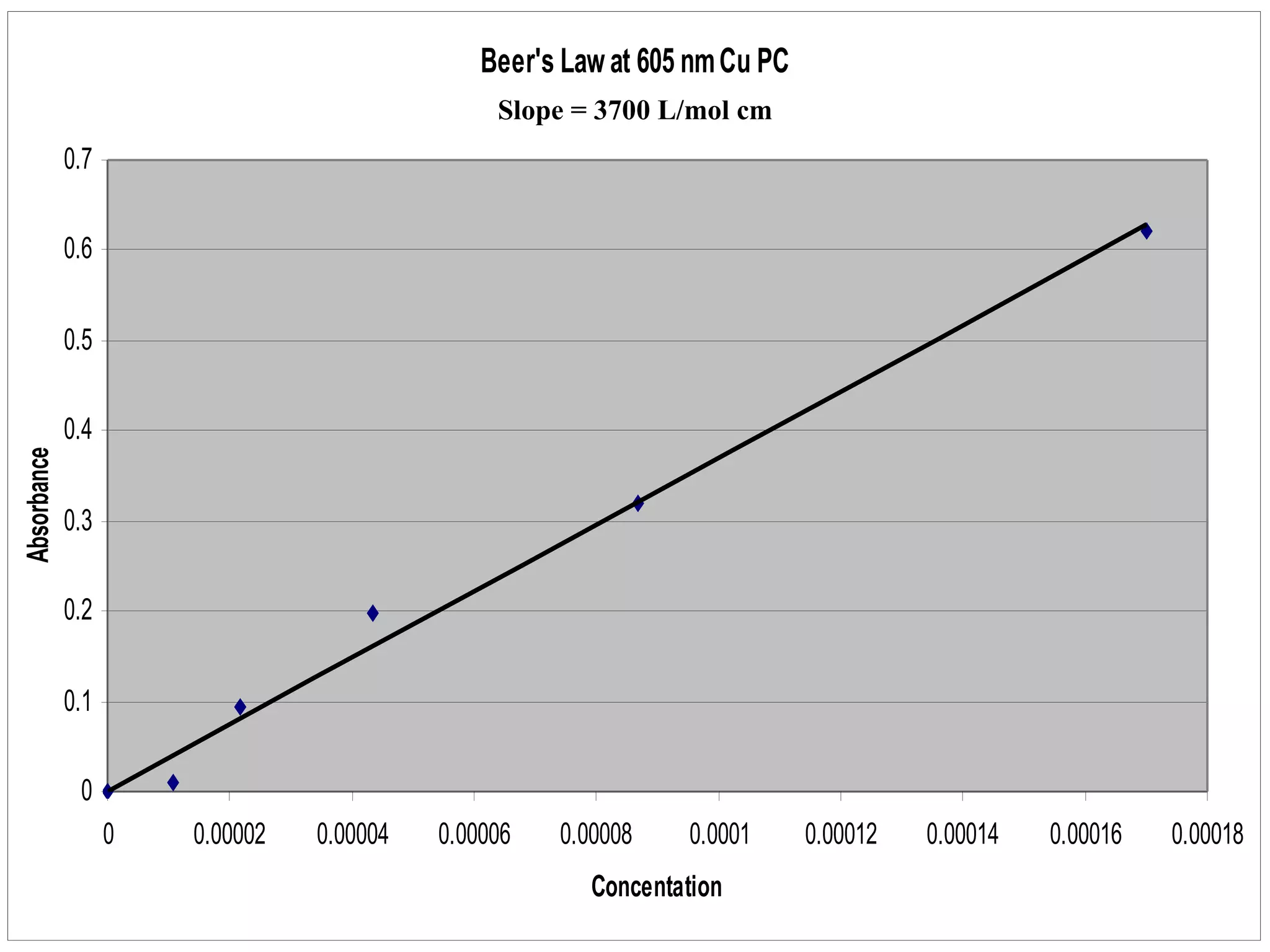
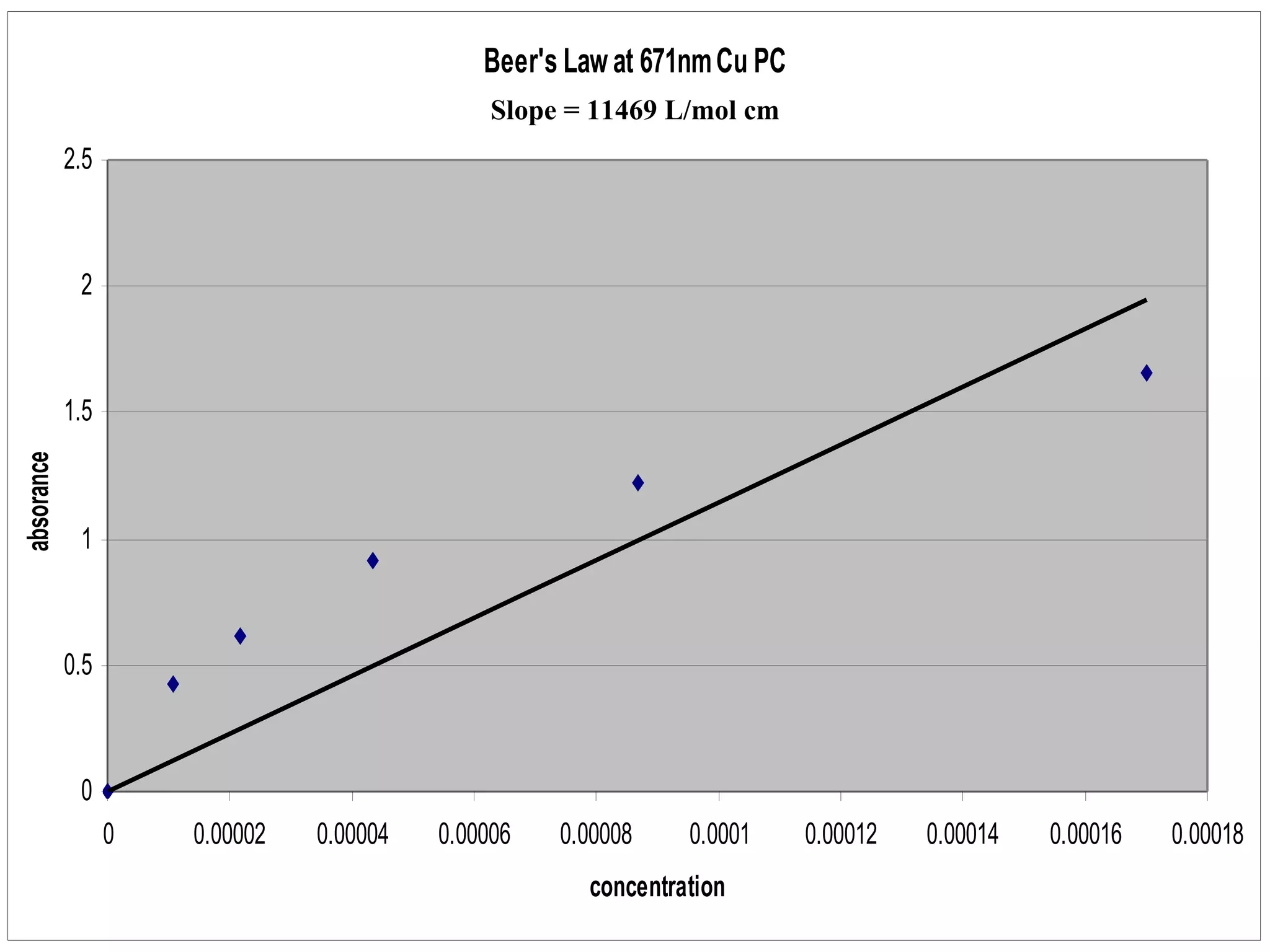
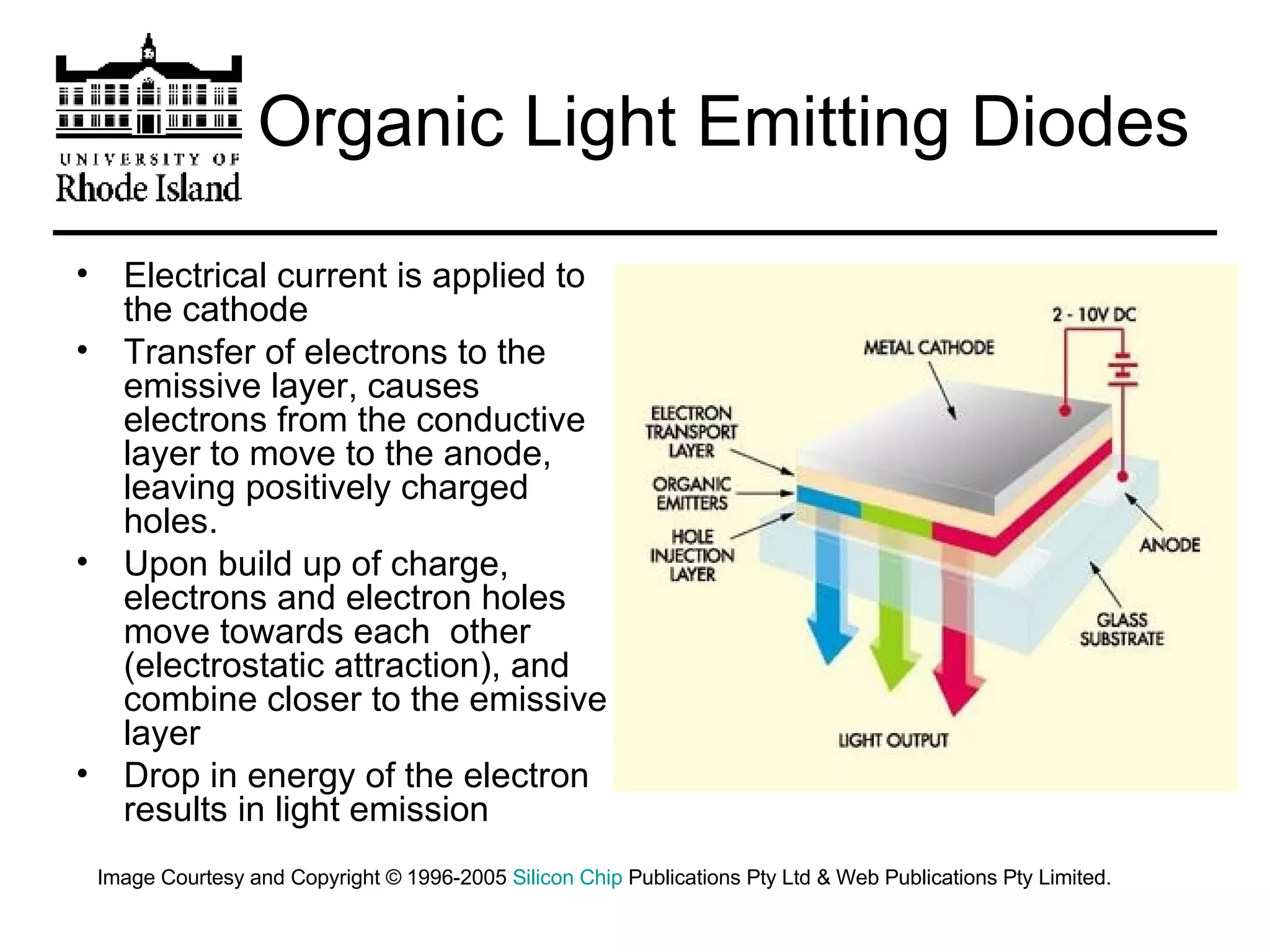
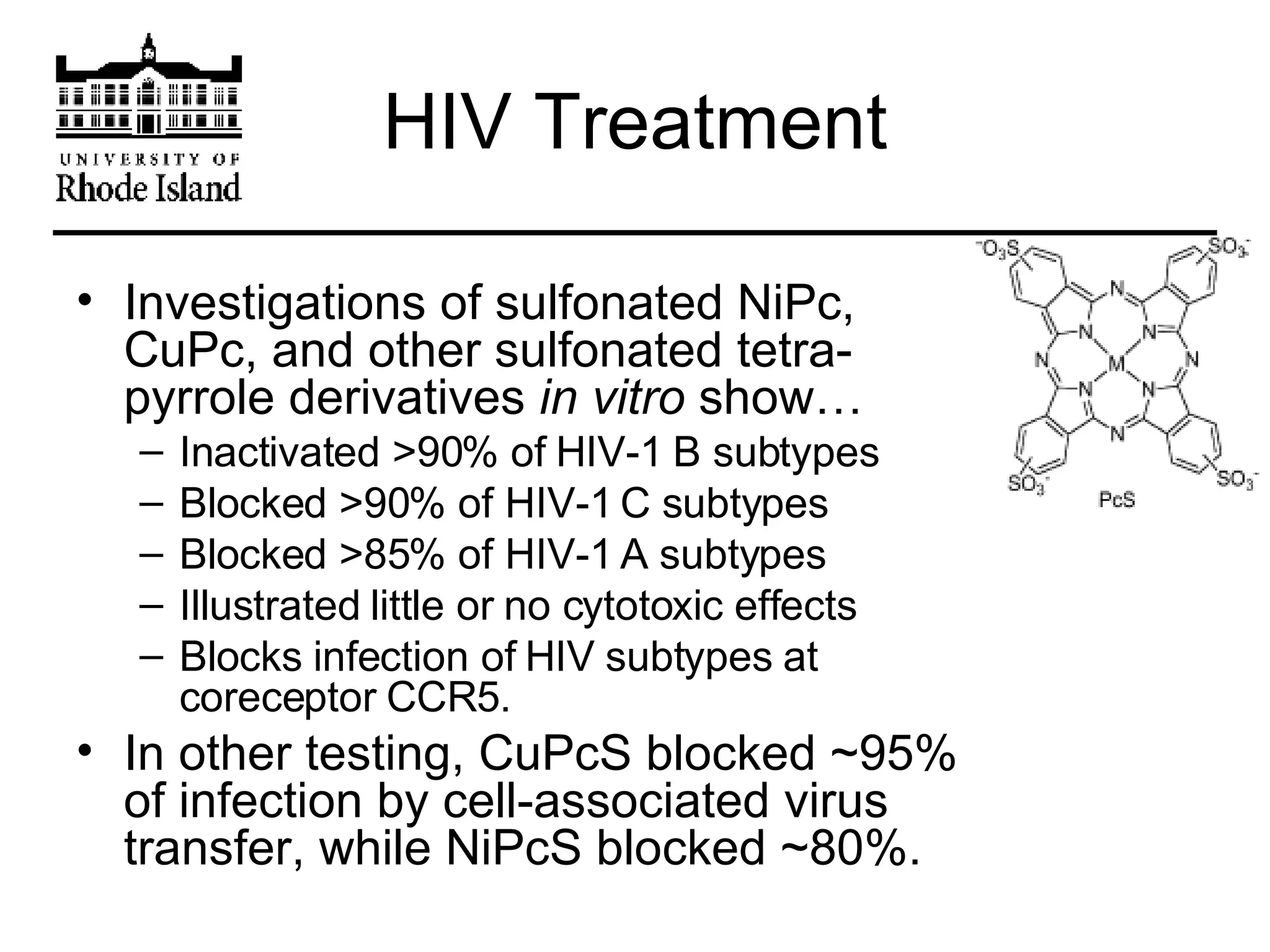
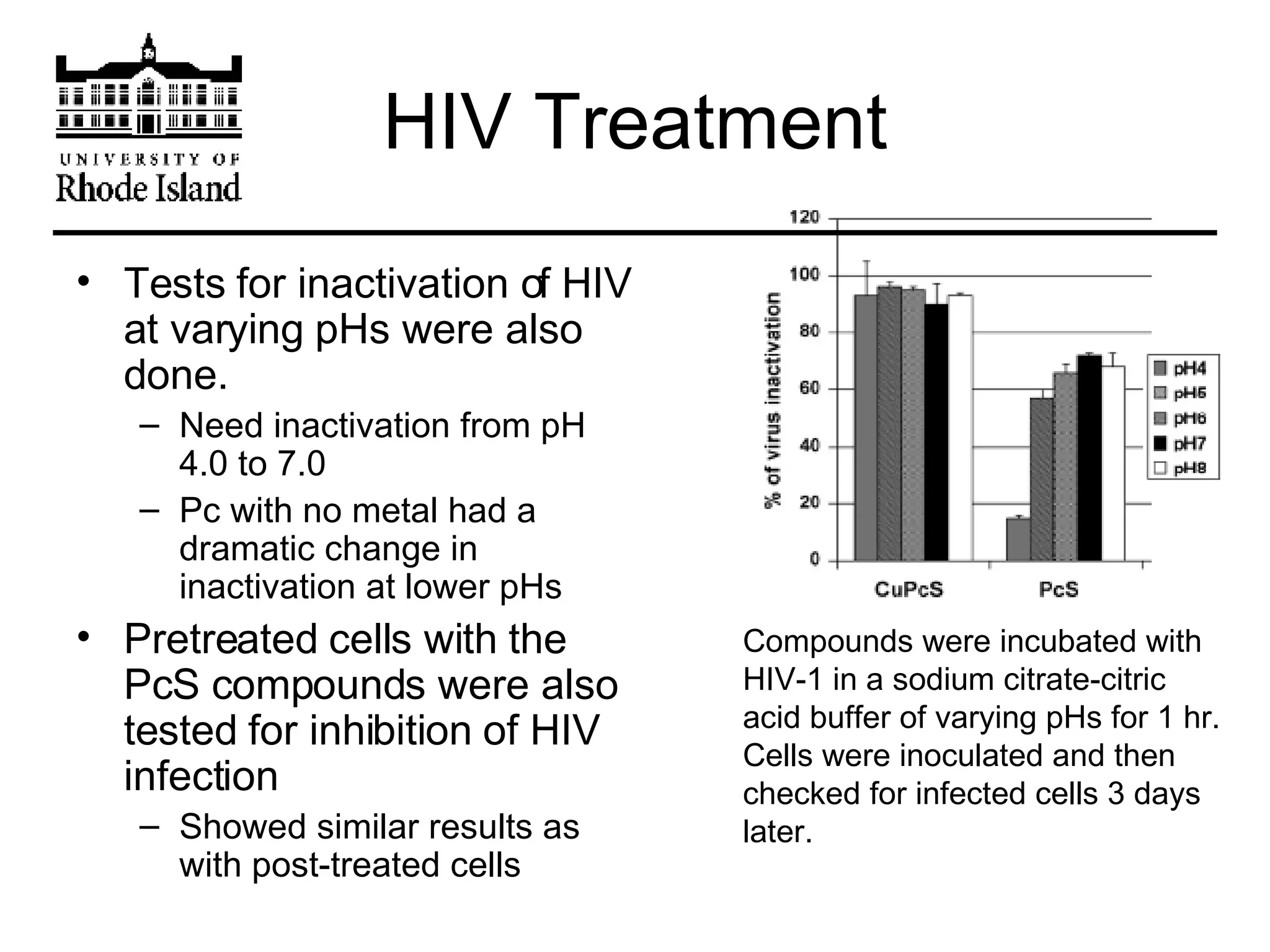
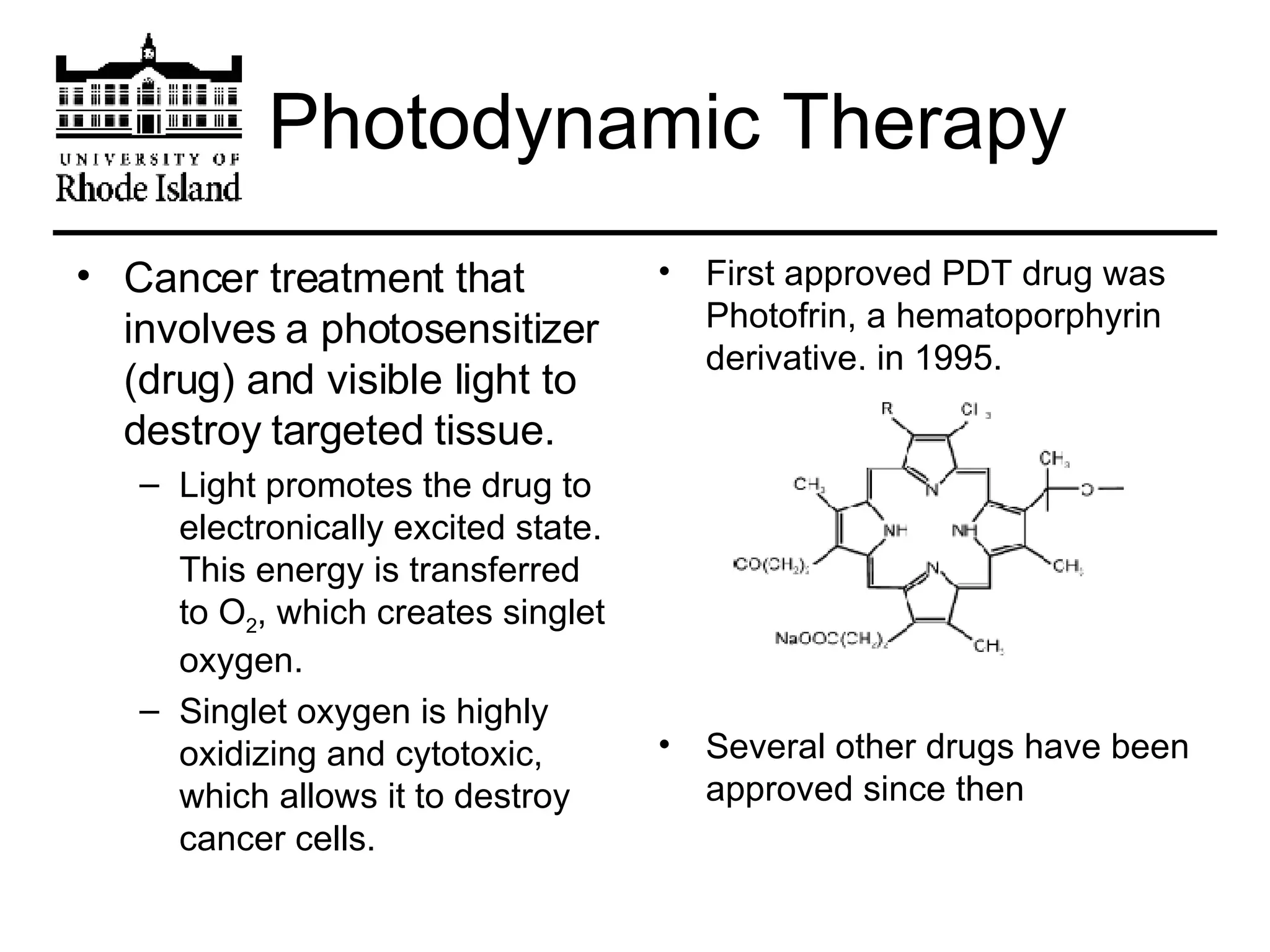
The document provides an overview of phthalocyanine metal cation complexes, including their history, applications, synthesis, and analysis methods. Phthalocyanines are stable planar molecules formed by reacting phthalanonitrile with metals at high temperatures. They were accidentally discovered in 1907 and further developed in the 1920s-1930s. Common applications include blue and green pigments, CD-R dyes, electrochemical uses, and as catalysts. Synthesis involves refluxing phthalonitrile with various metal salts. Characterization techniques like IR, UV-Vis, and EPR spectroscopy are used to analyze the products.