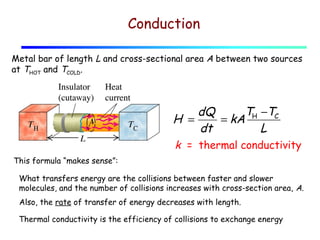
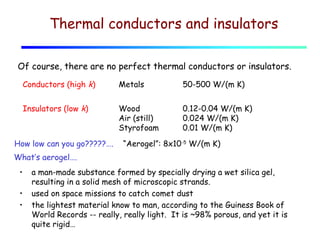
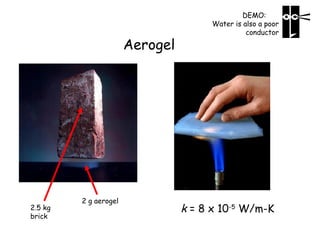
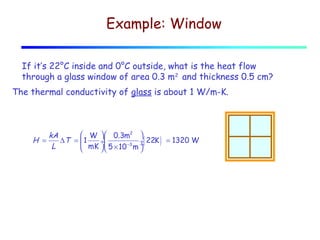
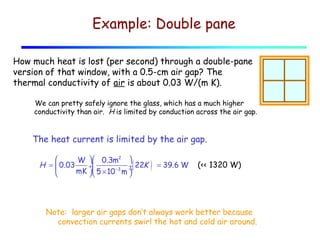
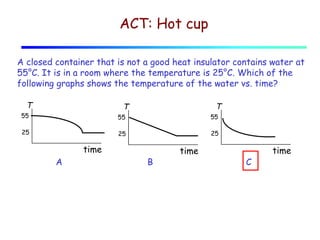
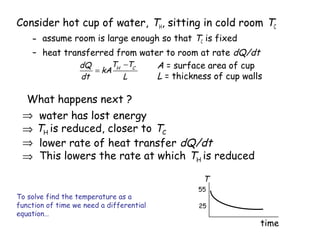
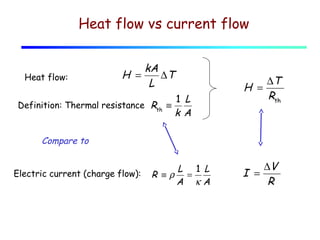
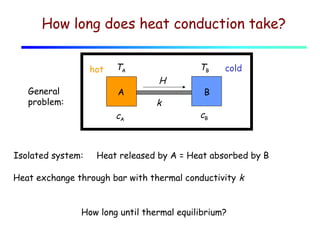
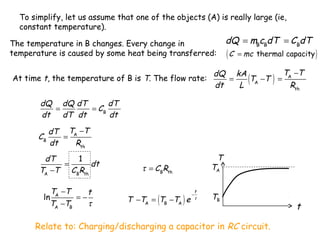
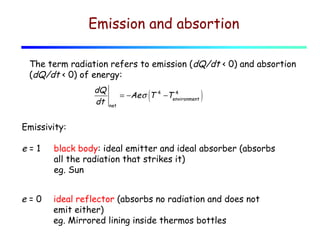
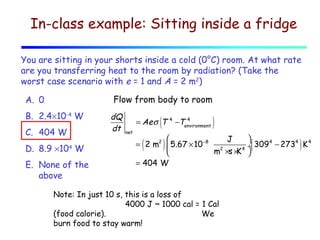
Heat can be transferred through three mechanisms: conduction, convection, and radiation. Conduction involves the transfer of heat between objects in direct contact through collisions of molecules. Convection involves the transfer of heat by the movement of fluids like gases and liquids. Radiation involves the emission and absorption of electromagnetic waves and can occur through a vacuum. The rate of heat transfer by conduction follows Fourier's Law and depends on factors like thermal conductivity, area, and temperature difference. Materials with high thermal conductivity like metals are good conductors while materials with low conductivity like wood and air are good insulators. Radiation transfer follows the Stefan-Boltzmann law and depends on emissivity, area, and the temperature difference between objects.