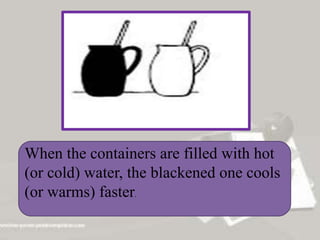
This document discusses applications of heat transfer in three areas:
a) Keeping homes insulated to reduce heating and cooling costs by inhibiting heat transfer through walls. Insulation materials have high R-values which reduce the rate of heat flow.
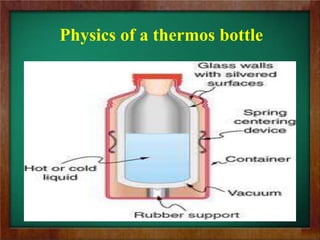
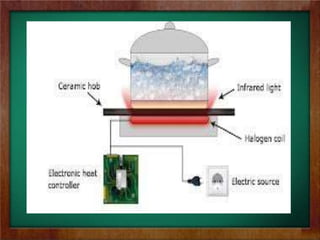
b) Examples of heat transfer in technology include regulating temperatures in orbiting satellites, the physics of how thermos bottles keep liquids hot or cold through insulation, and how halogen cooktop stoves absorb radiant energy.
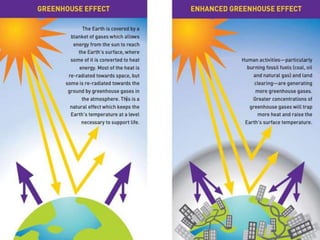
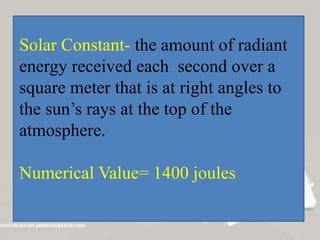
c) The greenhouse effect is a natural process that warms the Earth through the atmosphere absorbing and re-radiating solar energy trapped by greenhouse gases like carbon dioxide and methane. This summary outlines key applications and concepts related to heat transfer.