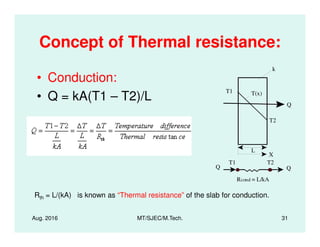
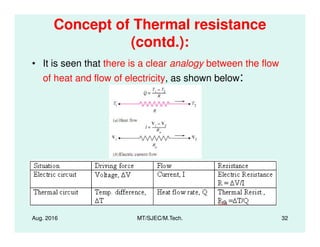
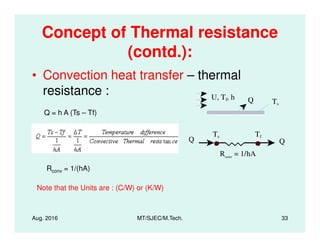
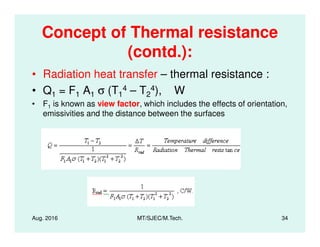
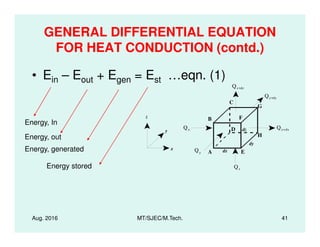
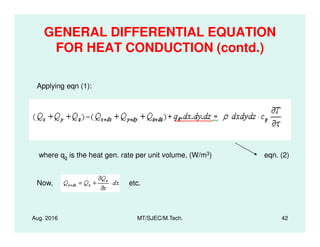
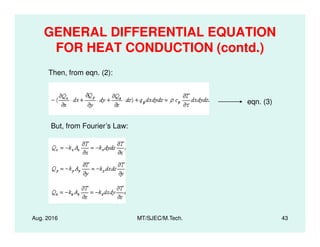
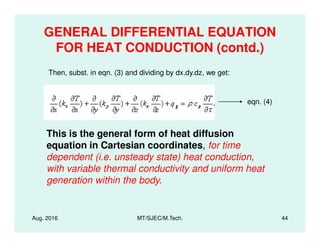
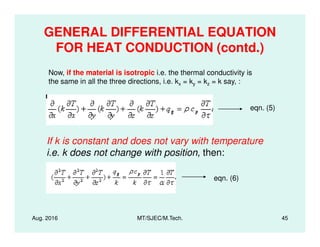
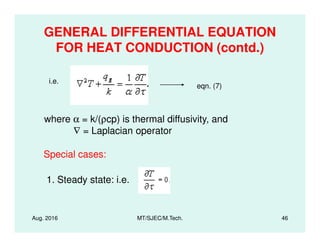
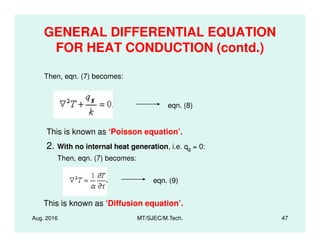
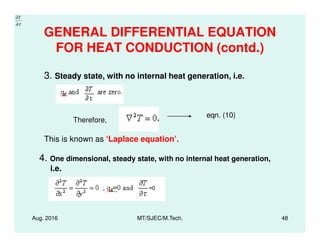
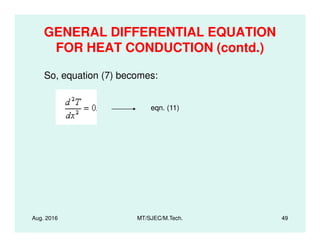
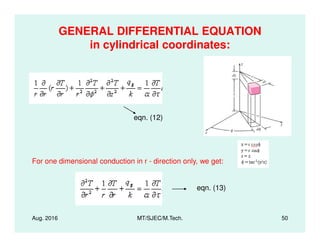
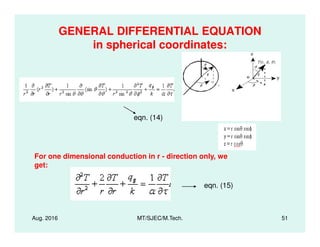
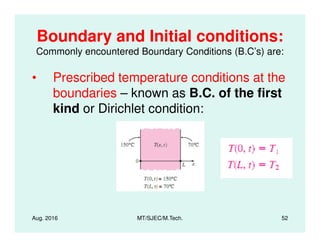
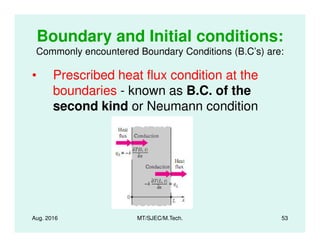
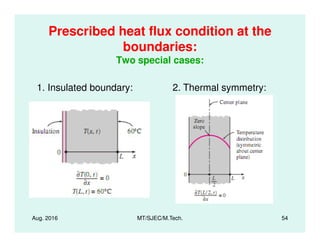
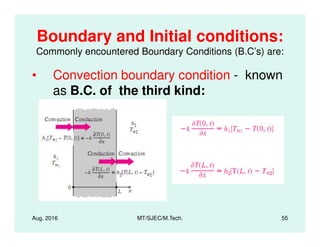
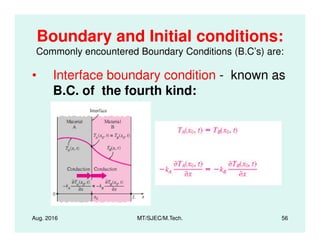
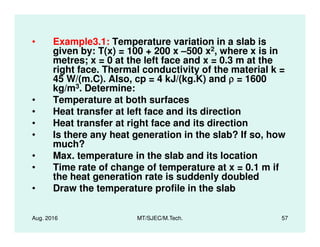
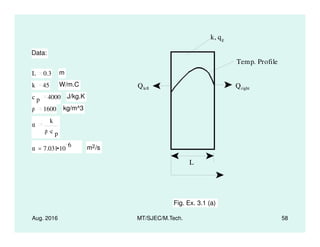
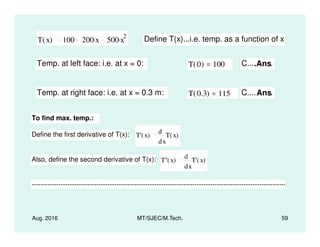
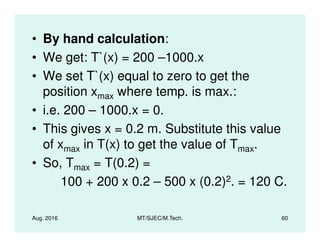
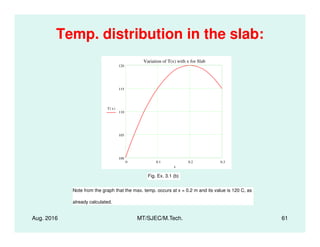
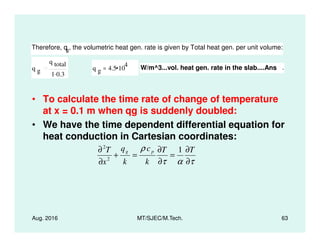
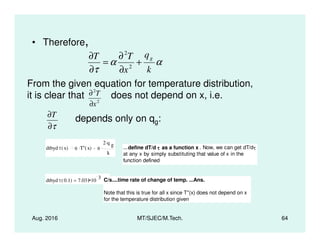
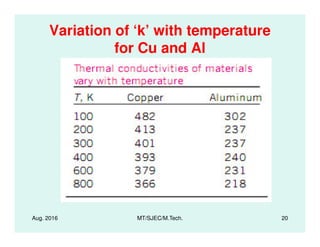
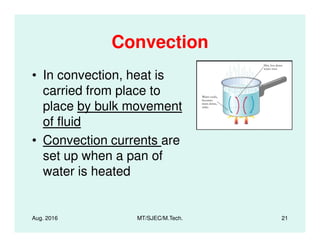
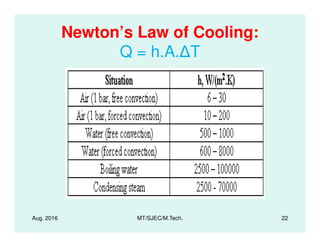
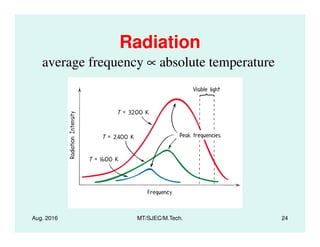
This document presents lectures on heat transfer fundamentals by Dr. M. Thirumaleshwar, aimed at M.Tech. students in Mechanical Engineering. It covers the introduction, fundamental laws, modes of heat transfer, applications across various engineering fields, and relevant equations. The material is intended to aid students and professionals in understanding heat transfer concepts and preparing for examinations.








![Radiation Heat Transfer
between bodies
• If the surrounding is at TS then the net
power radiated is:
P = A [ T4 - TS
4]
• Assuming on a dark, dry, night, T = 3 K:
Aug. 2016 MT/SJEC/M.Tech. 30
• Assuming on a dark, dry, night, TS = 3 K:
• Frost may form even if air temperature >
0 C since radiation cools the surface
faster than conduction heat lost from the
ground or air.](https://image.slidesharecdn.com/lectures-on-heattransfer-introduction-fundamentals-160828121740/85/Lectures-on-Heat-Transfer-Introduction-Applications-Fundamentals-Governing-Laws-30-320.jpg)