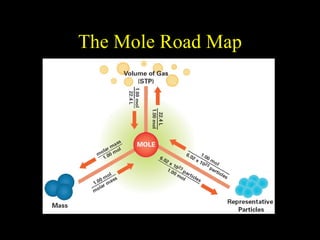
The document discusses moles, molar volume, and calculations involving moles of gases. It states that at standard temperature and pressure (STP), 1 mole of any gas occupies a volume of 22.4 L. It provides examples of calculating the number of moles or volume of a gas given amounts or volumes at STP. These include finding the cost of 2 moles of gold at $23.77 per gram, the grams of Be(NO3)2 for 3.2 moles, the volume of 0.60 moles of a gas at STP, and the moles of oxygen in 44.8 L at STP.