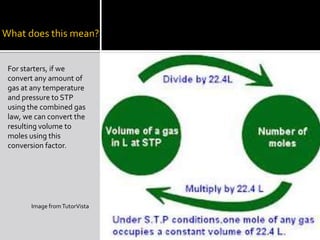
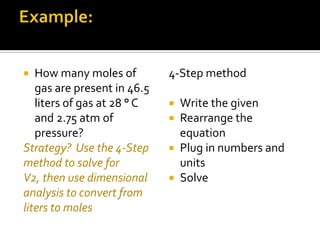
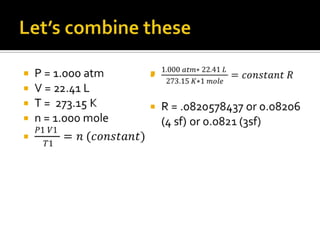
This document summarizes key points from Chapter 11 of Holt Modern Chemistry regarding Avogadro's law and relating the volume and number of moles of gases. It provides an example problem of calculating the number of moles of gas in 46.5 liters at 28°C and 2.75 atm by converting to STP conditions. The document notes how the combined gas law can be used to convert volumes to moles using the definition that 1 mole of any gas at STP occupies 22.414 liters. It also suggests developing a conversion factor or "multiplier" to more directly calculate moles without intermediate steps. Finally, it directs the reader to practice problems #40-52 at the end of chapter 11.