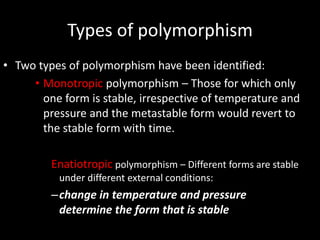
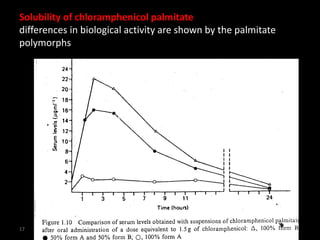
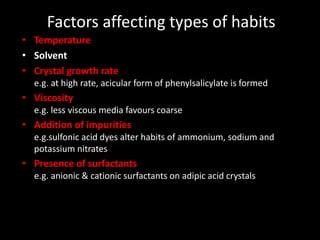
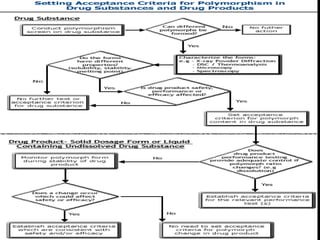
Polymorphism refers to different crystalline forms of the same substance that have different molecular arrangements and conformations. There are three main types of polymorphism: packing, conformational, and pseudopolymorphism (due to hydration or solvation). Polymorphs can be monotropic, where only one form is stable, or enantiotropic, where different forms are stable under different conditions. Monotropic polymorphs often have different melting points and properties. Polymorphism can impact drug properties like stability, dissolution, and bioavailability, so it is important to control the polymorphic form during drug development and manufacturing.