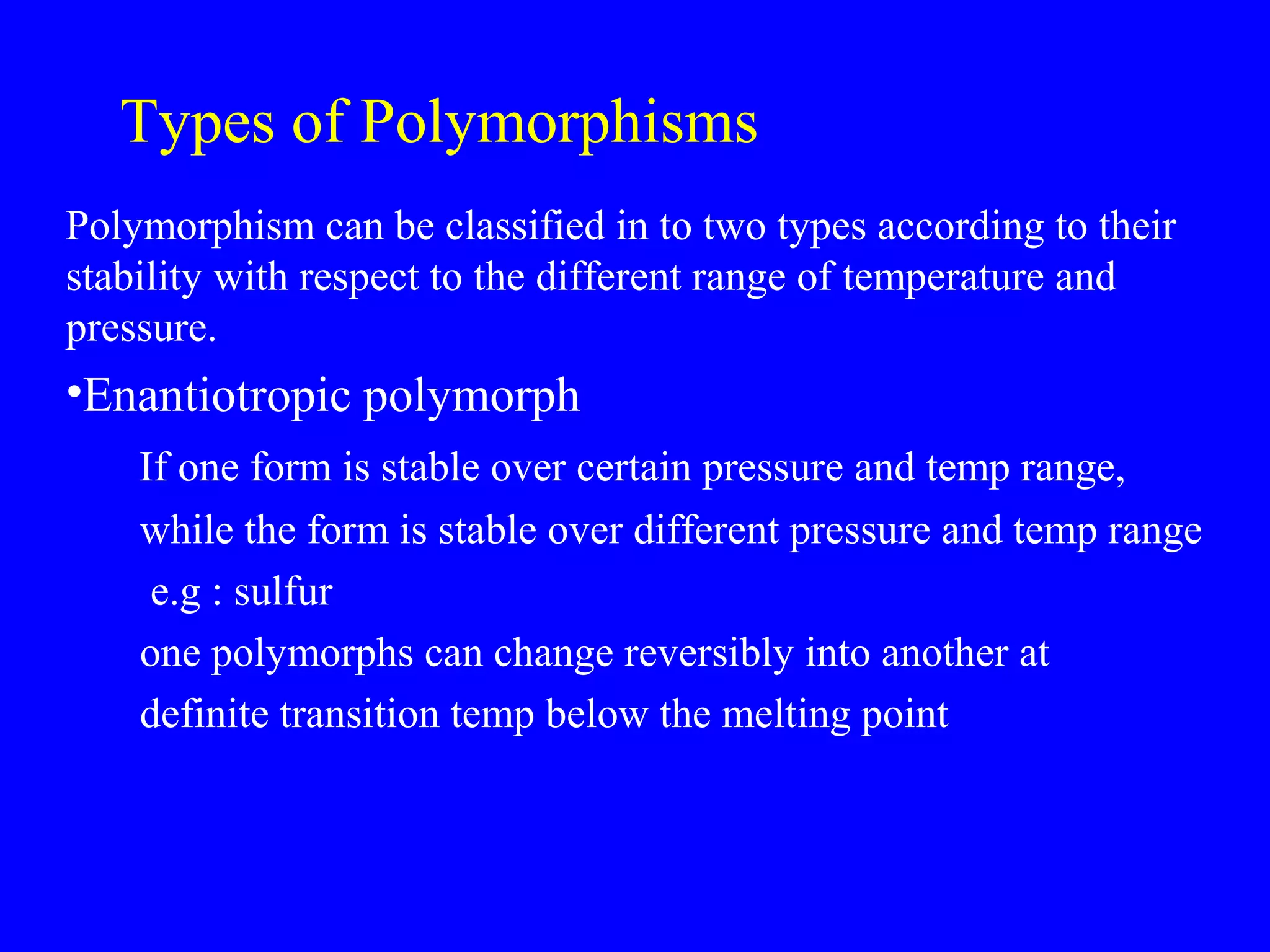
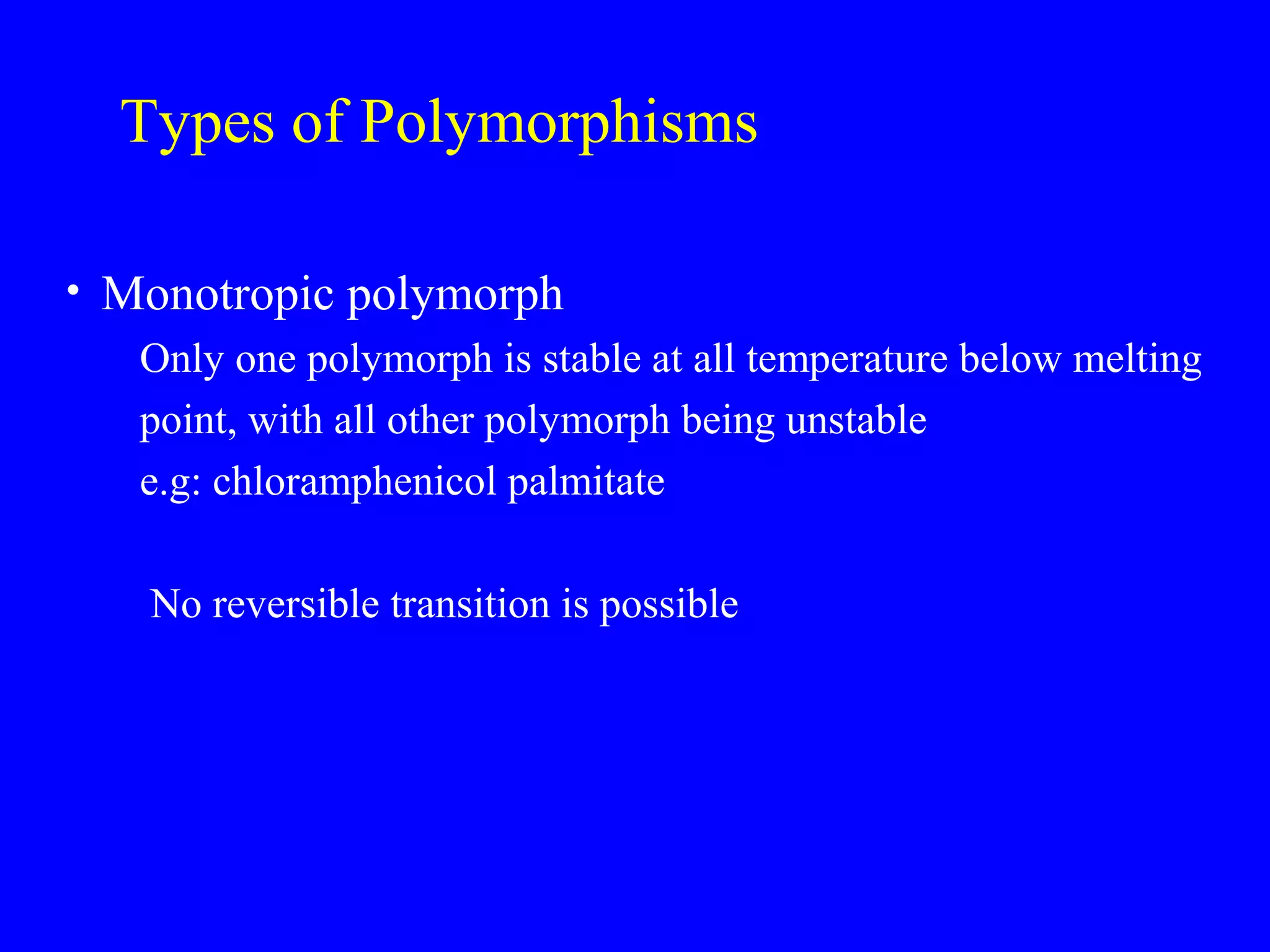
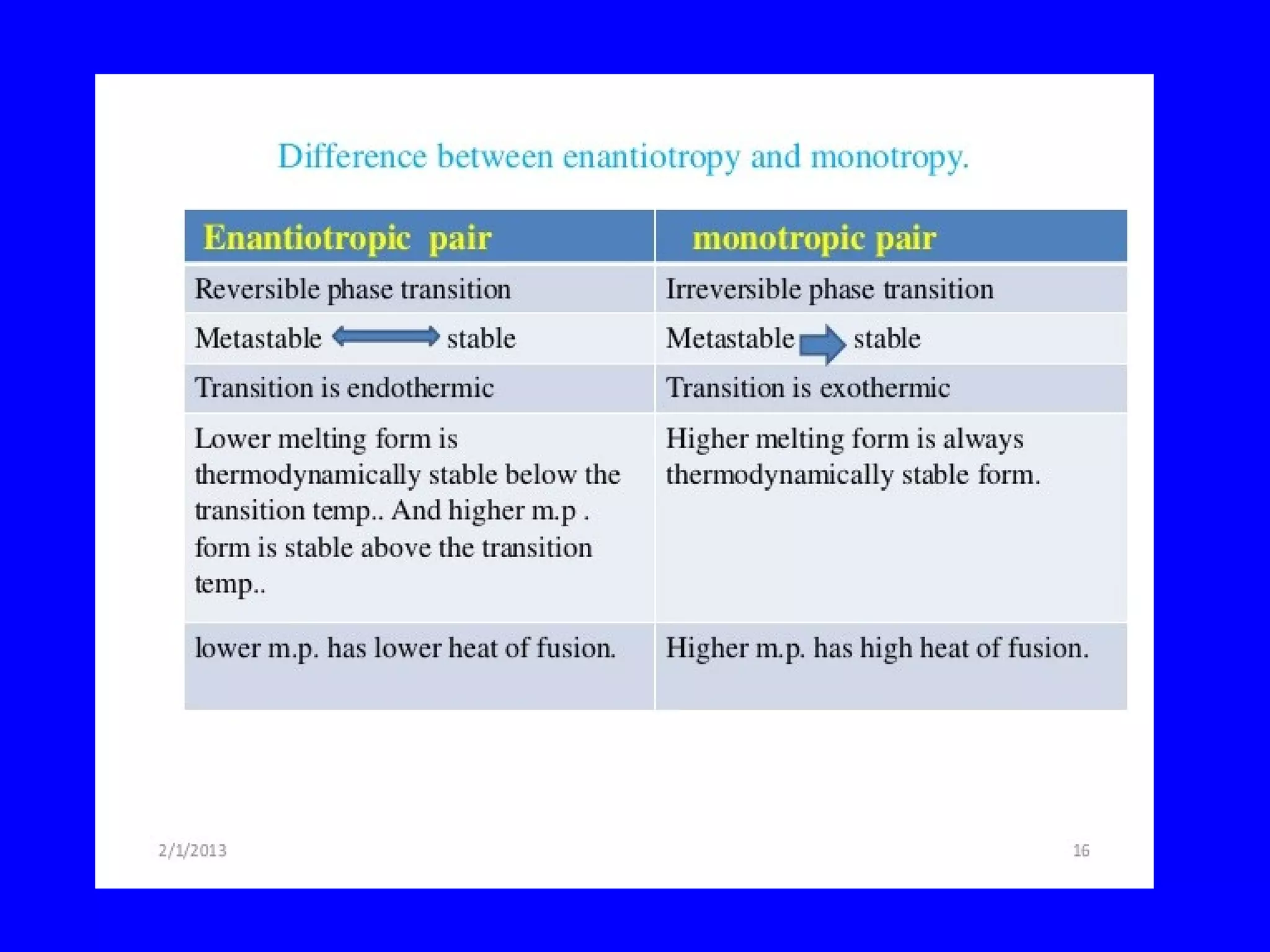
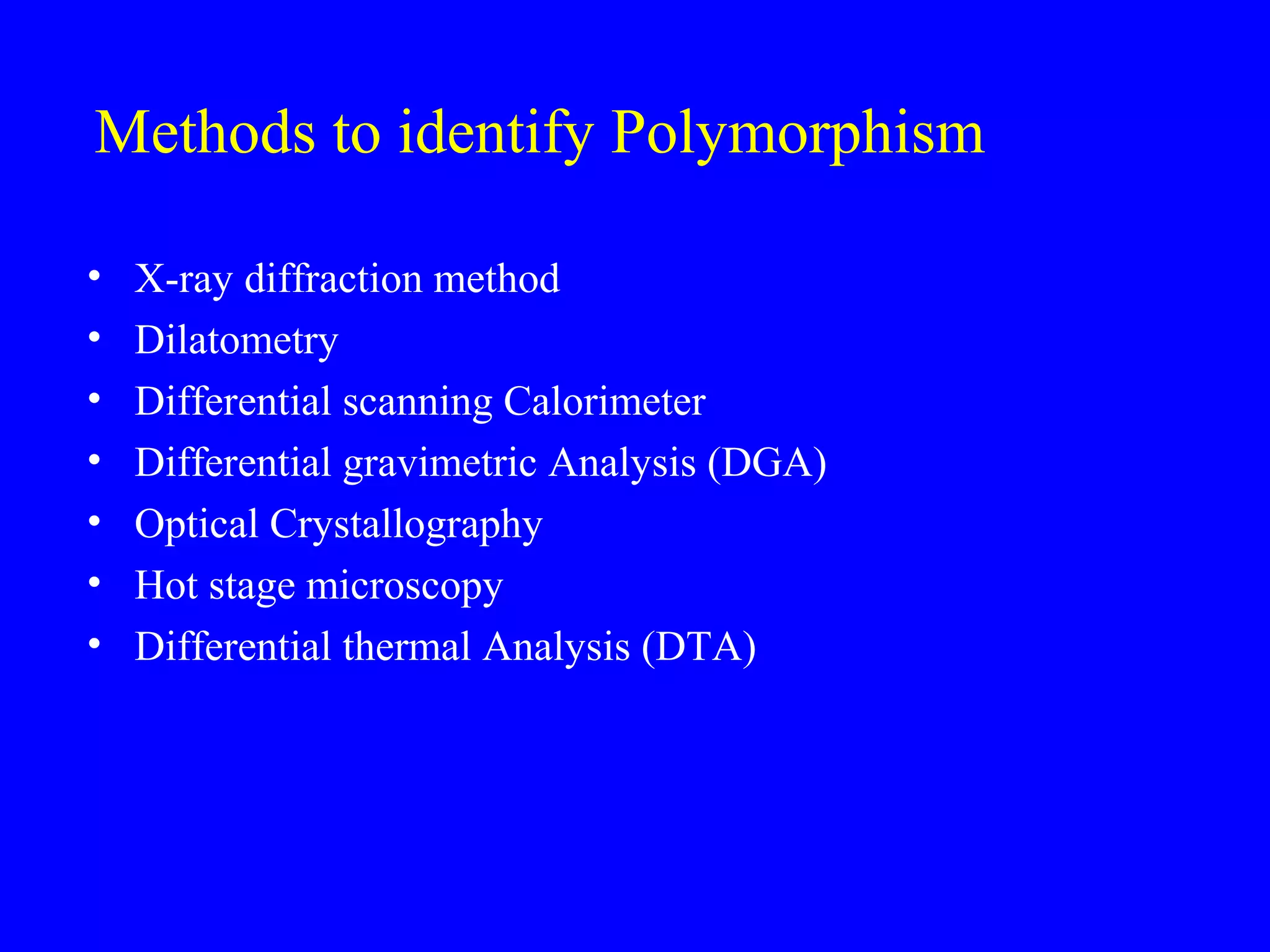
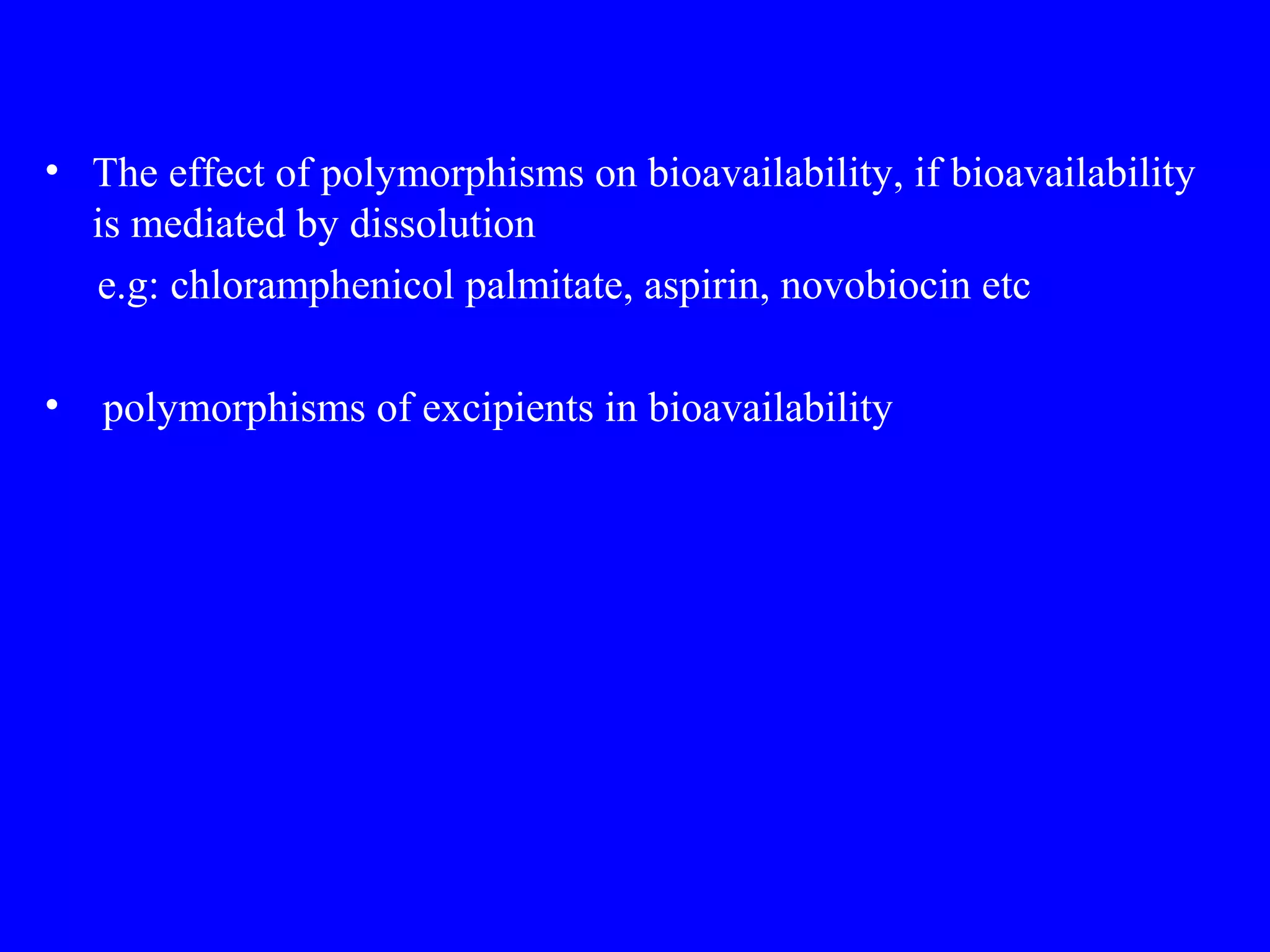
Polymorphism refers to when a substance exists in more than one crystalline form due to different arrangements of molecules in the crystal lattice. Over 50% of active pharmaceutical ingredients exhibit polymorphism. Common examples include sulfur and paracetamol. Polymorphic forms can differ in physical properties like solubility, melting point, stability, and dissolution rate. One form may be stable, while others are metastable. Polymorphism is classified as enantiotropic, where forms reversibly change below melting point, or monotropic, where only one form is stable below melting point. Identification methods include X-ray diffraction and thermal analysis. Polymorphism influences properties important for drug performance like flowability, dissolution, and







![References:
1 Raza K, Kumar P, Ratan S, Malik R, Arora S (2014)
Polymorphism: The Phenomenon Affecting the
Performance of Drugs. SOJ Pharm Pharm Sci, 1(2), 10.
•Grant DJW. [Chapter 1]. Theory and origin of
polymorphism. Polymorphism in pharmaceutical solids. In:
Brittain Harry G, editor. Marcel Dekker, Inc; 1999.
•www.wikkipedia.com](https://image.slidesharecdn.com/polymorphism-170429083703/75/Polymorphism-13-2048.jpg)
