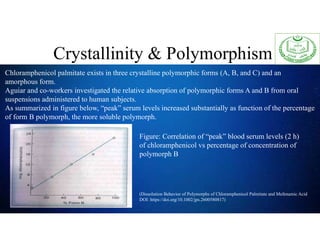
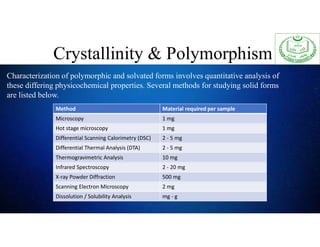
The document discusses the concepts of crystallinity and polymorphism in pharmaceuticals, detailing the characteristics and differences between crystalline and amorphous states. It covers the implications of these properties on drug stability, solubility, and bioavailability, as well as methods for their analysis and characterization. Furthermore, case studies illustrate the impact of polymorphic forms on drug solubility and formulation efficiency.