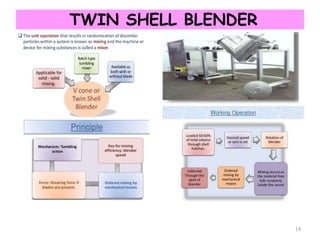
Mixing involves the combination of components through agitation to achieve uniformity, enhancing reactions and improving dissolution rates. The process is influenced by various factors and types of mixing mechanisms such as convective, shear, diffusive, bulk transport, and flow types. Proper mixing is crucial for product quality and reducing operational costs.