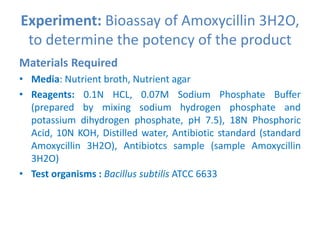
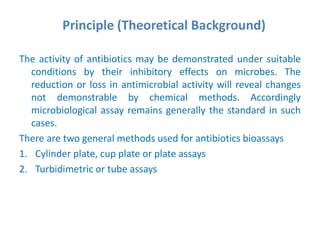
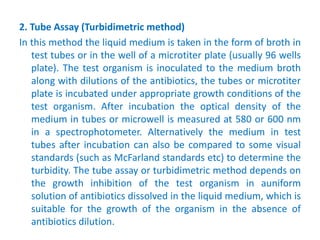
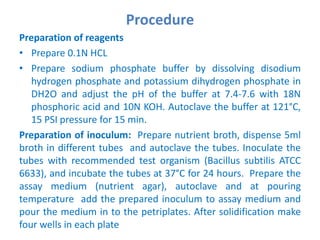
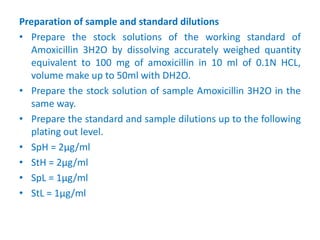
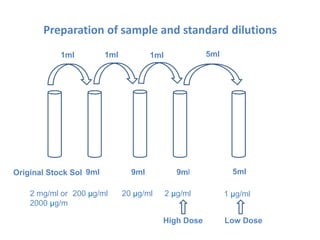
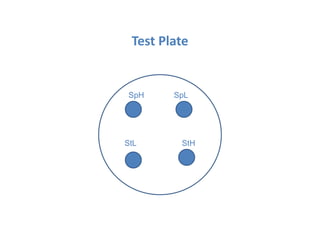
The document describes an experiment to determine the potency of amoxycillin 3H2O using a bioassay. The experiment uses Bacillus subtilis and involves preparing standard and sample dilutions of amoxycillin to test using cylinder plate assays. Zones of inhibition are measured after incubating the plates and potency is calculated using a formula comparing the sample and standard results.




![Results Interpretation
Calculate the potency of the antibiotic by the following formula
𝑃𝑜𝑡𝑒𝑛𝑐𝑦 𝑜𝑓 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 = 𝐴𝑛𝑡𝑖𝑙𝑜𝑔[
𝑆𝑝𝐻+𝑆𝑝𝐿 − 𝑆𝑡𝐻+𝑆𝑡𝐿
𝑆𝑝𝐻−𝑆𝑝𝐿 + 𝑆𝑡𝐻−𝑆𝑡𝐿
𝑋 𝐹] X 100
Where F= 0.301](https://image.slidesharecdn.com/labexperimentantibioticsbioassay-201016094936/85/Lab-experiment-antibiotics-bioassay-12-320.jpg)