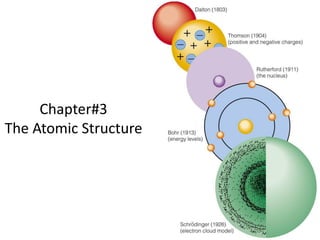
Atomic structure study notes about Mdcat
- 2. Discovery of Fundamental Particles of Atom • Proton • Electron • Neutron
- 3. The Greek Period In 2000 BC, The Greek Philosopher ‘Democritos’ gave a philosophy that all the substances are composed of very small particles called ‘Atomos’ means indivisible in Greek. The Greek rejected this concept and continued to believe in Aristotle’s concept of 4 basic elements i.e; fire, earth, water and earth.
- 4. Dalton’s Atomic Theory John Dalton was an English scientist who proposed this theory in 1808. Main Points of Dalton’s Atomic Theory: i) Matter is composed of indivisible particles called atom. ii) Identical atoms are known as elements. iii) Elements are combined chemically to form compound. iv) Chemical reaction is only the rearrangement of atoms and atoms themselves are not changed.
- 5. Discovery of Cathode Rays/Electrons Discharge Tube Experiment • Heinrich Giessler was a German Scientist who invented the discharge tube to pass electric current through air at low pressure. • W. Crookes was the scientist who discovered cathode rays through Discharge tube. • J. J. Thomson discovered the properties of cathode rays and concluded that these rays are actually material particles. • Stoney named them Electrons.
- 7. Properties of Cathode Rays • Cathode rays travel in straight line so they cast sharp shadows of objects. • These rays evolve from cathode and moves to anode. • They can pass through thin foil. • They carry negative charge. • They can rotate a light paddle wheel hence they can exert mechanical pressure and they have kinetic energy. • Cathode rays consist of material particles known as electrons. • When cathode rays strike with a meta they produce X-rays. • Cathode rays does not depend upon the gas filled in the discharge tube. • The e/m ratio of cathode rays remain same for all the gases.
- 8. Discovery of Protons Discharge Tube Experiment Protons/Anode Rays/Positive Rays/Canal Rays Were discovered by Goldstein by using a discharge tube with perforated cathode.
- 9. Properties of Anode Rays • Anode rays travel in straight line so they cast sharp shadows of objects. • These rays evolve from anode and moves to cathode. • They cannot pass through thin foil. • They carry positive charge. • Anode rays consist of material particles known as protons and it is 1836 times heavier than electrons. • Anode rays depend upon the gas filled in the discharge tube. • The e/m ratio of cathode rays is different for all the gases. So, Heavier atoms have lower e/m values.
- 10. NOTE • After the discovery of electron and proton, scientists gave their atomic models before the discovery of neutron. • But for the sake of rhythm we will study about the discovery of radioactivity and neutron.
- 11. Radioactivity The spontaneous disintegration of the unstable nucleus of an atom in the form of invisible radiation is called radioactivity. There are two types of radioactivity: i) Natural radioactivity (Elements after Pb 82) Discovered by Henry Bacquerel. i) Artifical Radioactivity (Bombardment of particles) Discovered by James Chadwick for discovery of neutron. Radioactivity is a nuclear reaction: 92U238 90Th234 + 2He4
- 12. • Henry Bacquerel discovered the phenomena of radioactivity by the emission of beta particles through pitch blende. • Madam Curie & Perrie Curie discovered that the emission of particles is due to Radium. • Rutherford discovered that there are three types of radioactive rays. • James Chadwick discovered artificial radioactivity through which he discovered neutron.
- 13. Types of Radioactive Rays
- 14. Properties of Radioactive Rays Property α - Particles - Particles - rays Nature Helium nucleus electrons Electromagnetic radiations Mass 4 amu 9.11x10-31 kg 0 Charge Positive Negative No charge Velocity < light ≈ light = light Penetration Power Least (1 – 2 cm in air) More than alpha (1 – 2 m in air) Most (15 – 20 cm in lead) Ionisation Power highest medium least
- 16. Discovery of Neutron Artificial Radioactivity James Chadwich (1934) 4Be9 + 2He4 6C12 + 0n1 neutrons can penetrate more than gamma rays.
- 17. Summary Particle Discoverd by Method Mass in kg Charge in C Electron W. Crookes Discharge Tube 9.11x10-31 kg -1.6x10-19 C Proton Goldstein Discharge Tube 1.67x10-27 kg 1.6x10-19 C Neutron James Chadwick Aritificial Radioactivity 1.76x10-27 kg 0
- 18. Thomson’s Plum Pudding Model
- 19. Rutherford’s Atomic Model (Nuclear Model of Atom) Rutherford bombarded alpha particles from polonium metal into the thin gold foil. Gold was selected because of bigger atoms and extremely malleable. A photographic plate was also present behind the gold foil. Through this experiment he concluded that:
- 21. Defects in Rutherford’s Atomic Model • If electron is emitting energy continuously then it must stop afterwards and falls into the nucleus making a spiral path. • If energy is emitted by atom in the continuous manner then we should get a continuous spectrum but in contrast to it we get a line spectra.
- 22. Spectra (band of light) Plural (spectrum) The dispersion of light when it is passed through a prism is called spectrum. There are two types of spectra: 1) Line Spectra 2) Continuous Spectra
- 23. Continuous Specta (Formed by white light) Red is the least deviated light having the highest wavelength (7000 Å), lowest frequency and lowest energy. Violet is the most deviated light having the lowest wavelength (4000 Å), highest frequency and highest energy.
- 24. Line Spectra / Atomic Spectra (Emitted by atoms in discharge tube)
- 25. Planck’s Quantum Theory Proposed by Max Planck Quanta = packets of energy • Energy is released from atom in discontinuous manner in the form of packets of energy called quanta. Quanta of light is called photon. • The energy of emitted light is directly proportional to its frequency. E=hυ Here h=Planck’s constant (6.625x10-34 JS) (6.625x10-27 erg. S) This theory proves that energy is quantized.
- 26. Bohr’s Atomic Model (Solar System Model of Atom) • Based on Max Planck’s Quantum Theory. • Neils Bohr removed the defects of Rutherfor’s atomic model. Main Points: i) As soon as atom revolves in its specified energy level it neither radiates not absorbs energy. ii) When it jumps from lower energy level to higher energy level it absorbs energy and when it jumps back to lower energy level it absorbs energy. iii) The energy is absorbed and released in the form of quanta. iv) The change in energy can be calculated by: ΔE=E2-E1=hυ v) Only those orbits are possible where the angular momentum of electron is the integral multiple of h/2π mvr = nh/2 π
- 28. Radius of Hydrogen Atom By Neils Bohr 4 2 2 2 k mZe h n r r1=ao=0.529 Å ao is known as Bohr’s radius for hydrogen. rn=n2 x ao
- 29. Energy of an electron 1 2 3 . 1313 kJmol n En
- 30. Wave number (υ) The number of waves per unit length is called wave number. 2 2 2 1 1 1 n n RH Here, RH is known as Rydberg constant for hydrogen. The value of Rydberg constant is 109678 cm-1 n1 is the lower orbit. n2 is the higher orbit.
- 31. Hydrogen Spectrum • The following series of spectral lines are obtained by hydrogen gas. Lyman Series (when electron jumps to 1st orbit) UV region Balmer Series (when electron jumps to 2nd orbit) Visible region Paschen Series (when electron jumps to 3rdorbit) Infrared region Bracket Series (when electron jumps to 4th orbit) Far infrared region Brackett Series (when electron jumps to 5thorbit) Far infrared region
- 33. Defects in Bohr’s Atomic Model • Applicable only for the atoms with an electron in the last shell.
- 35. X-Ray and Atomic Number • Atomic Number discovered by Moseley.
- 36. Heisenberg’s Uncertainty Principle • Given by Werner Heisenberg in 1925. • It rejected Bohr’s circular orbit concept. “It is impossible to determine the exact position and momentum of an electron simultaneously in an atom.” mvr≈nh/2π
- 38. Quantum Mechanical Model Wave Mechanical Model (Ervine Schrodinger)
- 39. Orbit/Shell/Energy Level/Stationary State Orbital (i) The fixed path in which electron revolves. (i) The region around nucleus where the possibility of finding an electron is maximum. (ii) It is circular in shape. (ii) It exists in different shapes. (iii) It is discovered by Bohr. (iii) It is discovered by Schrodinger. (iv) An orbit contains 2 or more electrons. (iv) An orbital contains maximum two electrons. (v) The number of electrons can be calculated by 2n2 (vi) The number of electrons can be calculated by 2(2l+1)
- 40. Types of Orbitals • There are 4 types of orbitals. (i) s – orbital (s=sharp lines) (ii) p – orbital (p=principal lines) (iii) d – orbital (d=diffused lines) (iv) f – orbital (fundamental lines)
- 41. s – sub shell s - orbital • It is spherical in shape. • It can accommodate maximum 2 electrons. • There is no nodal plane in s-orbital. • It is non-directional.
- 42. p – subshell contains px py pz orbitals • It is dumb-bell in shape. • It contains 6 electrons in 3 different orbitals having different orientations. • It is directional. The electron charge density is maximum at the ends. • The nodal plane lies between the point of intersection of two nodes.
- 44. d – sub shell dxy dyz dzx dx2-y2 dz2 orbitals • It is double dumb-bell in shape. • There are 5 orbitals in d – subshell. • d – sub shell contains max 10 electrons.
- 45. f – sub shell • They have complex saucer like shape. • They contain maximum 14 electrons in 7 orientations.
- 46. Quantum Numbers • It tells about the address of electron in an atom. • There are four types of quantum numbers. (i) Principal Quantum number (n) orbit (ii) Azimuthal / subsidiary quantum number (l) sub-shell (iii) Magnetic quantum number (m) orbital (iii) Spin quantum number (s) electron
- 47. Principal Quantum Number • It tells about the number of orbit or the size of orbital. • It is denoted by ‘n’. • Its value is in natural number. • n=1 for K-shell, n=2 for L-shell etc.
- 49. Azimuthal Quantum Number • It tells about the shape of orbital. • Its value is in whole number (0, 1, 2, 3) • The value can be calculated by the formula l=0 to n-1
- 50. Magnetic Quantum Number • It tells about the orientation of orbital. • Its value is in integer. • For any value of l we have 2l+1 values of m. • For any value of l: m=-l 0 +l
- 51. Spin Quantum Number • It tells about the spin of electron in its own axis. • Its value is either +1/2 or -1/2
- 53. Rules of Electronic Configuration • There are four rules of electronic configuration. (i) Pauli’s exclusion principle (ii) n+l rule (iii) Aufbau Principle (iv) Hund’s rule of maximum multiplicity
- 54. Definitions • Atomic Radius • Ionization Potential • Electron Affinity • Electronegativity