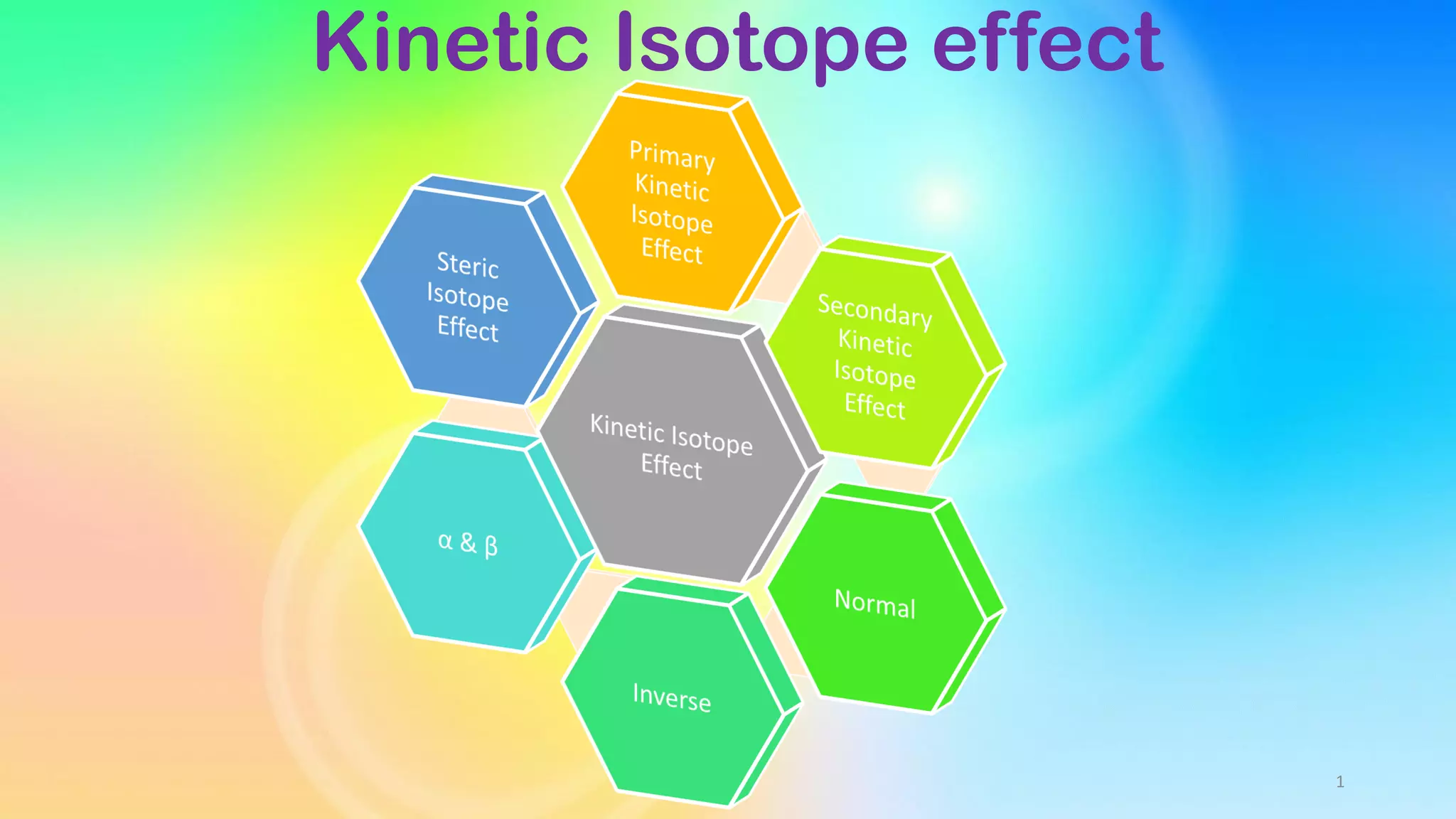
1) Kinetic isotope effects occur when the rate of a chemical reaction is different for molecules that only differ in their isotopic substitution. This is because isotopes can alter vibrational zero-point energies.
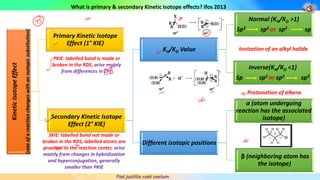
2) Primary kinetic isotope effects involve bonds made or broken in the rate-determining step, arising from differences in zero-point vibrational energies between reactants and transition states. Secondary effects involve isotopes near but not at the reaction center.
3) The magnitude of primary kinetic isotope effects depends on factors like the symmetry and geometry of the transition state, with more linear transition states giving larger effects. Secondary effects depend more on hybridization changes and hyperconjugation effects.