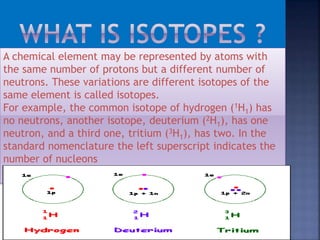
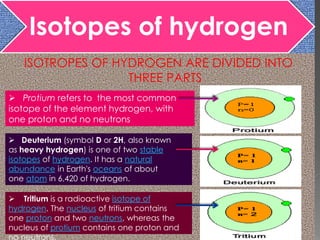
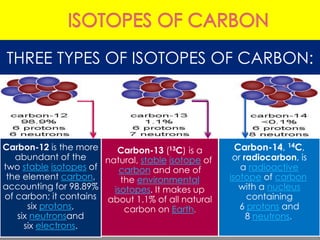
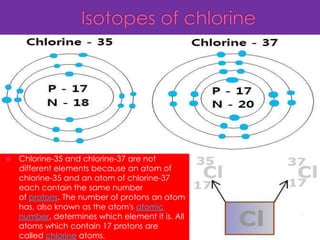
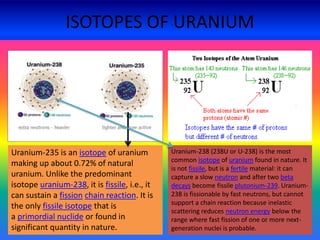
Three isotopes of hydrogen are discussed: protium, deuterium, and tritium. Protium is the most common isotope with one proton and no neutrons. Deuterium has one neutron and is a stable isotope found naturally. Tritium is radioactive with one proton and two neutrons. Three isotopes of carbon are also discussed: carbon-12, carbon-13, and carbon-14. Carbon-12 and carbon-13 are stable isotopes while carbon-14 is radioactive. The document also briefly discusses isotopes of chlorine, oxygen, uranium, and neon. The key properties of isotopes are that they have the same number of protons but different numbers of neutrons, and thus different