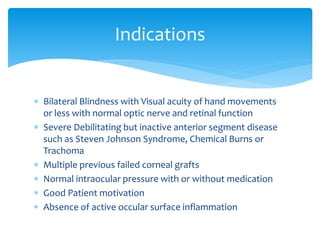
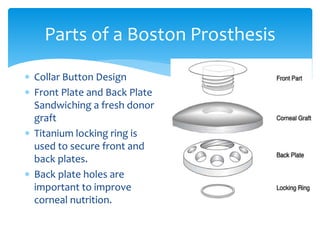
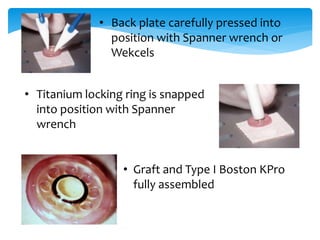
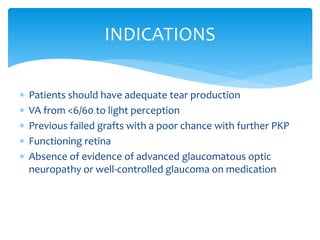
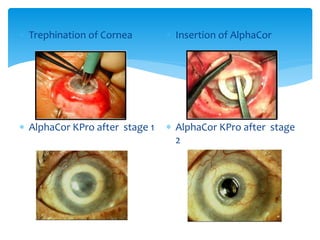
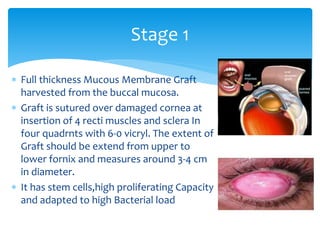
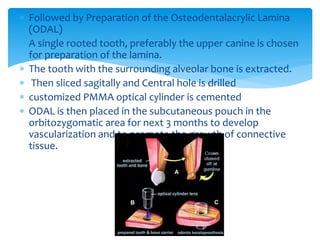
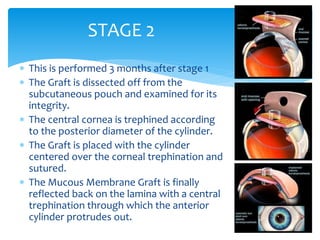
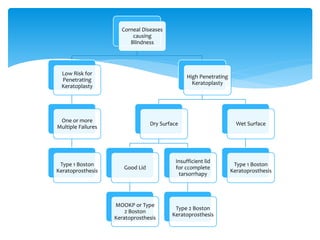
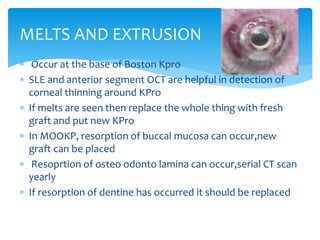
Keratoprosthesis is a surgical procedure where a severely damaged or diseased cornea is replaced with an artificial cornea to restore vision. The most commonly used procedure is the Boston Keratoprosthesis, which uses a donor corneal graft sandwiched between a front and back plate. Complications include melts and extrusion of the graft, infectious endophthalmitis, glaucoma, and retroprosthetic membranes. Close follow up is required after surgery to monitor for and manage any complications.