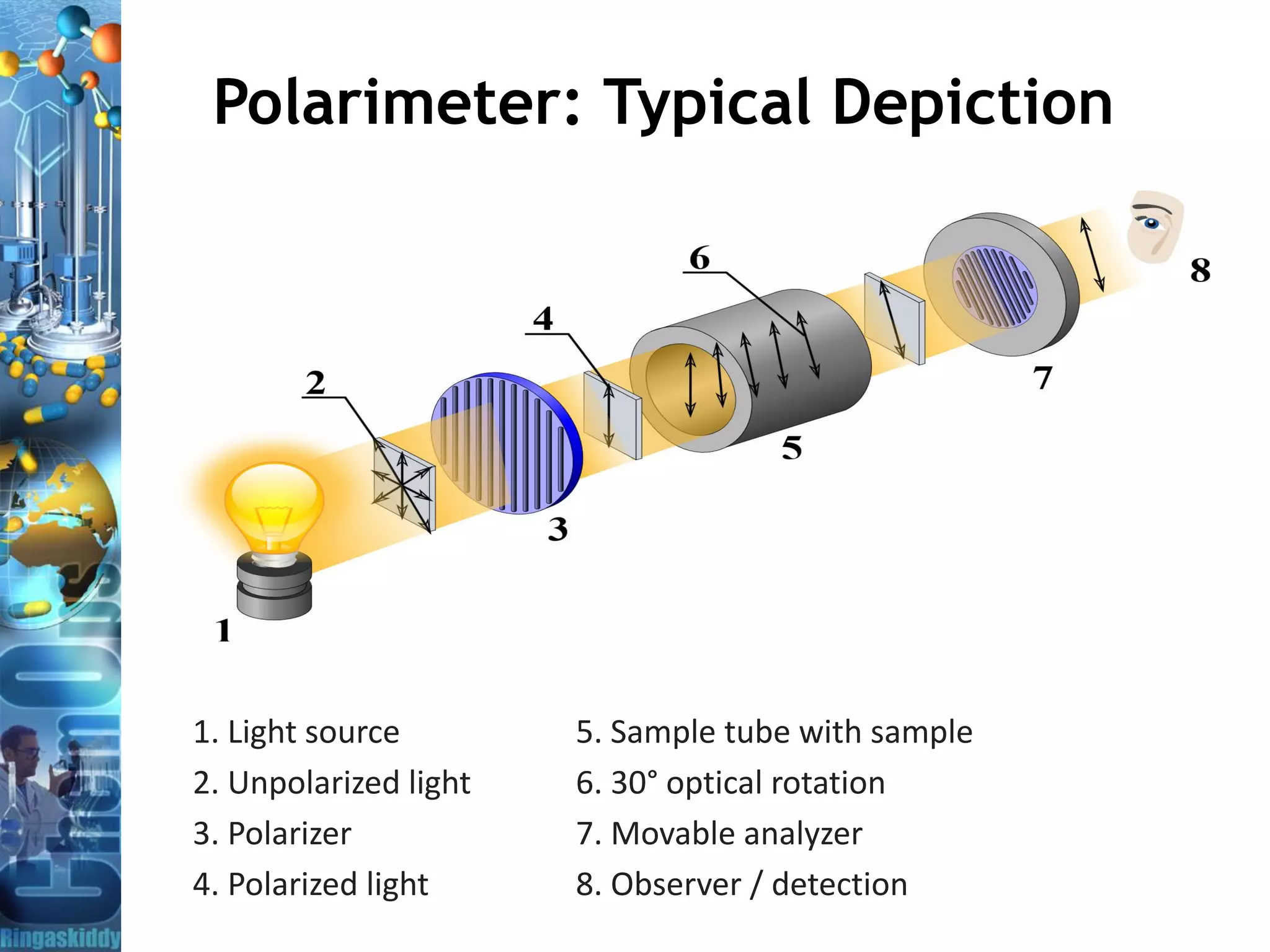
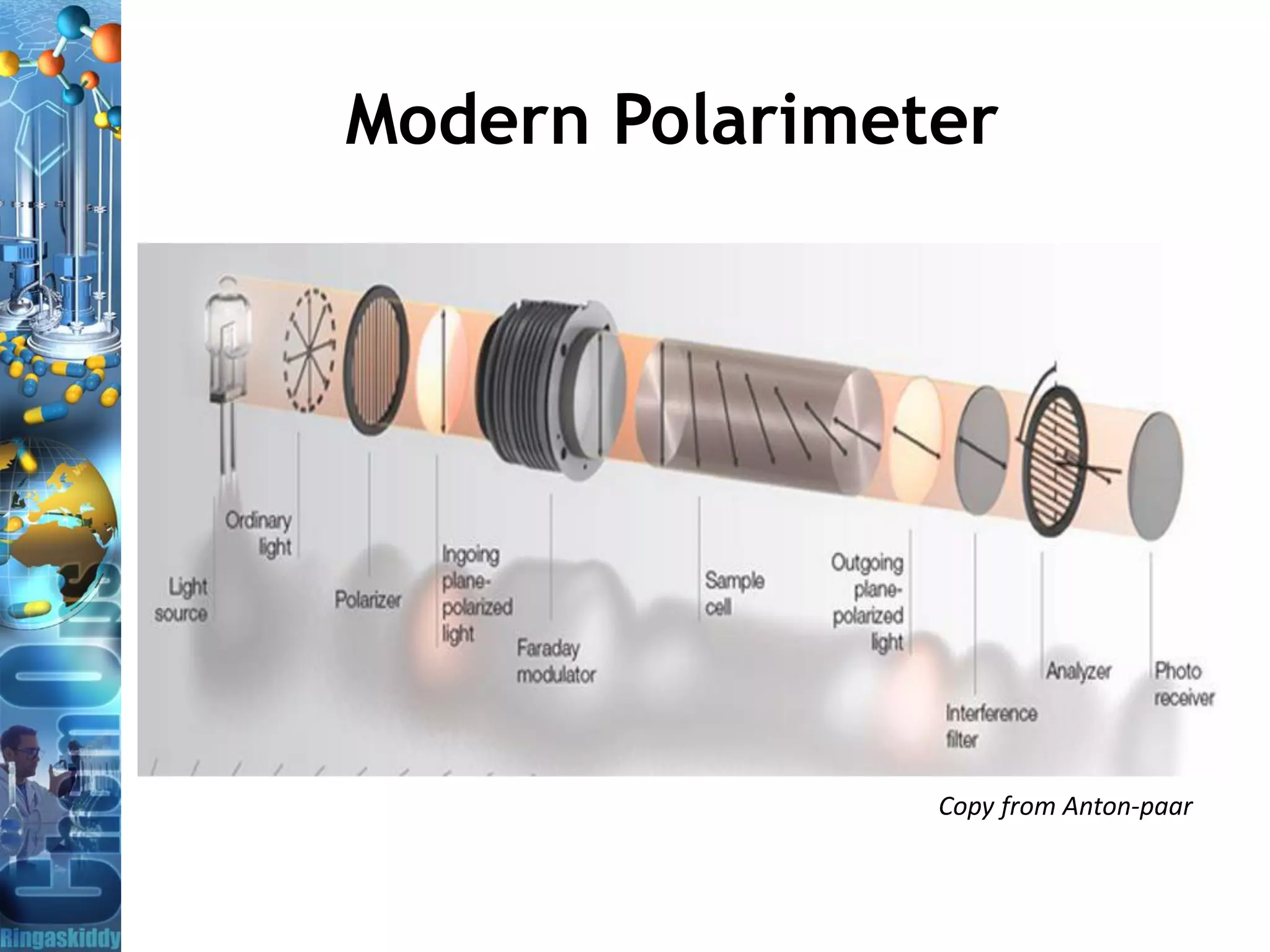
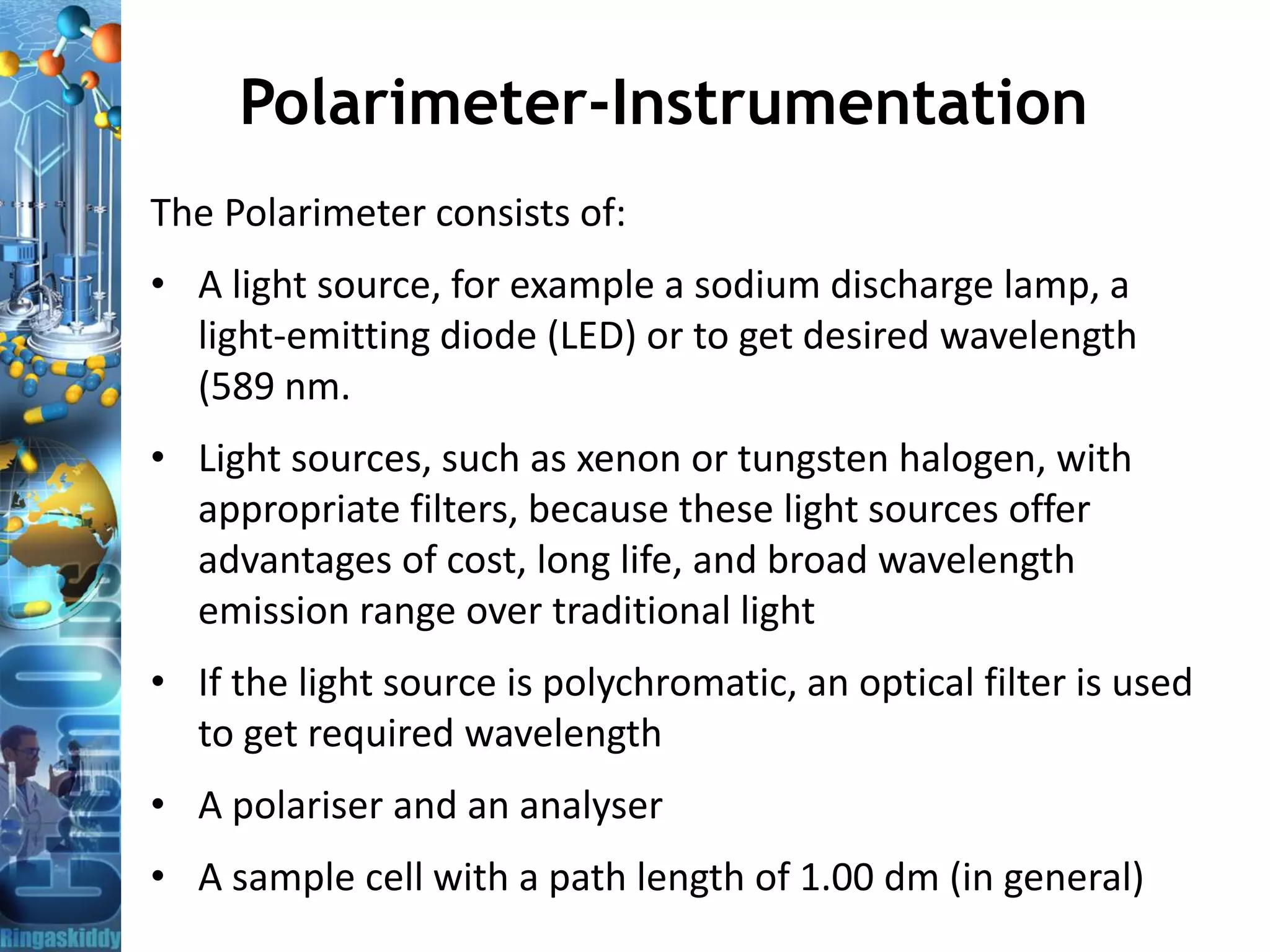
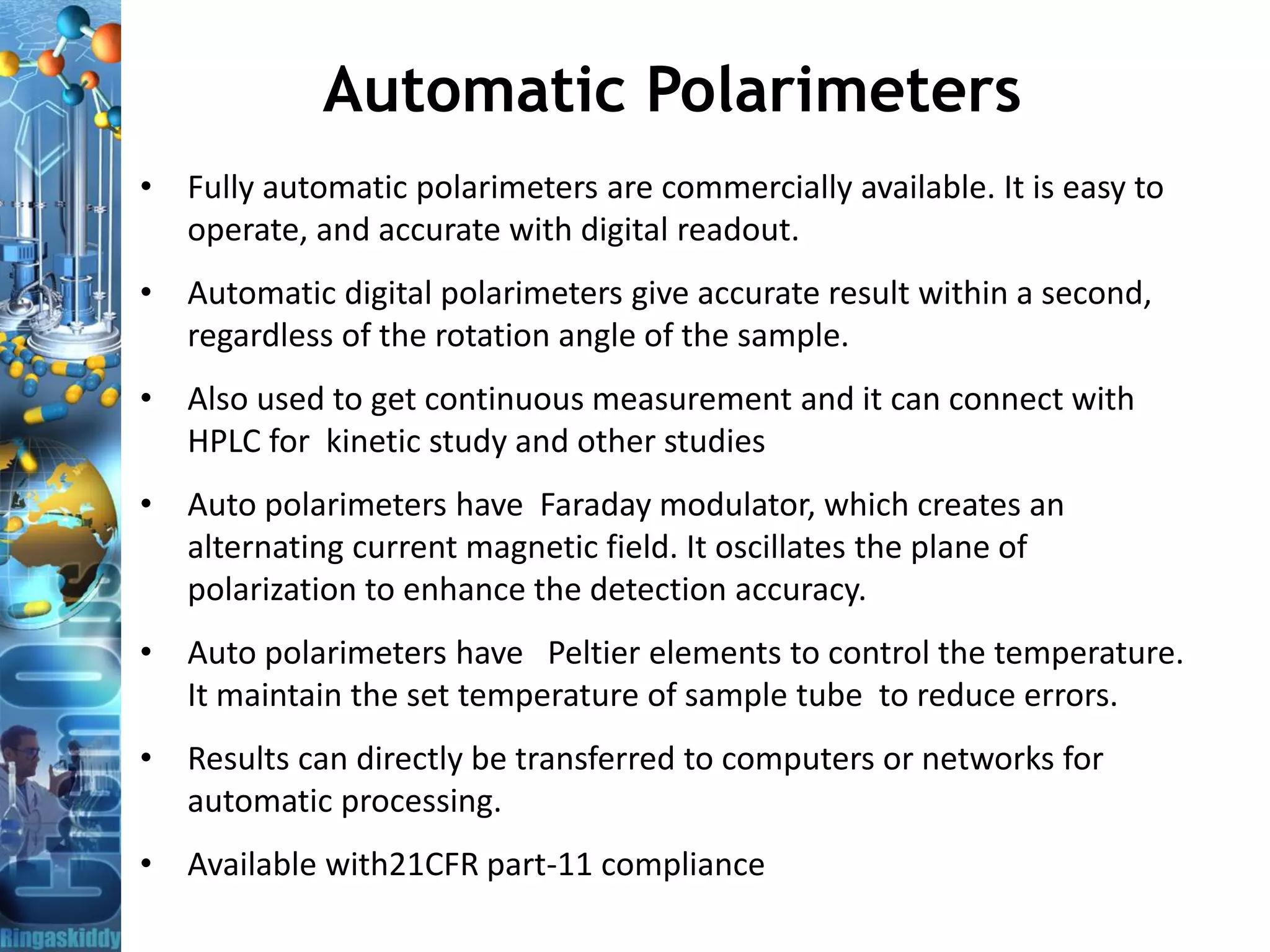
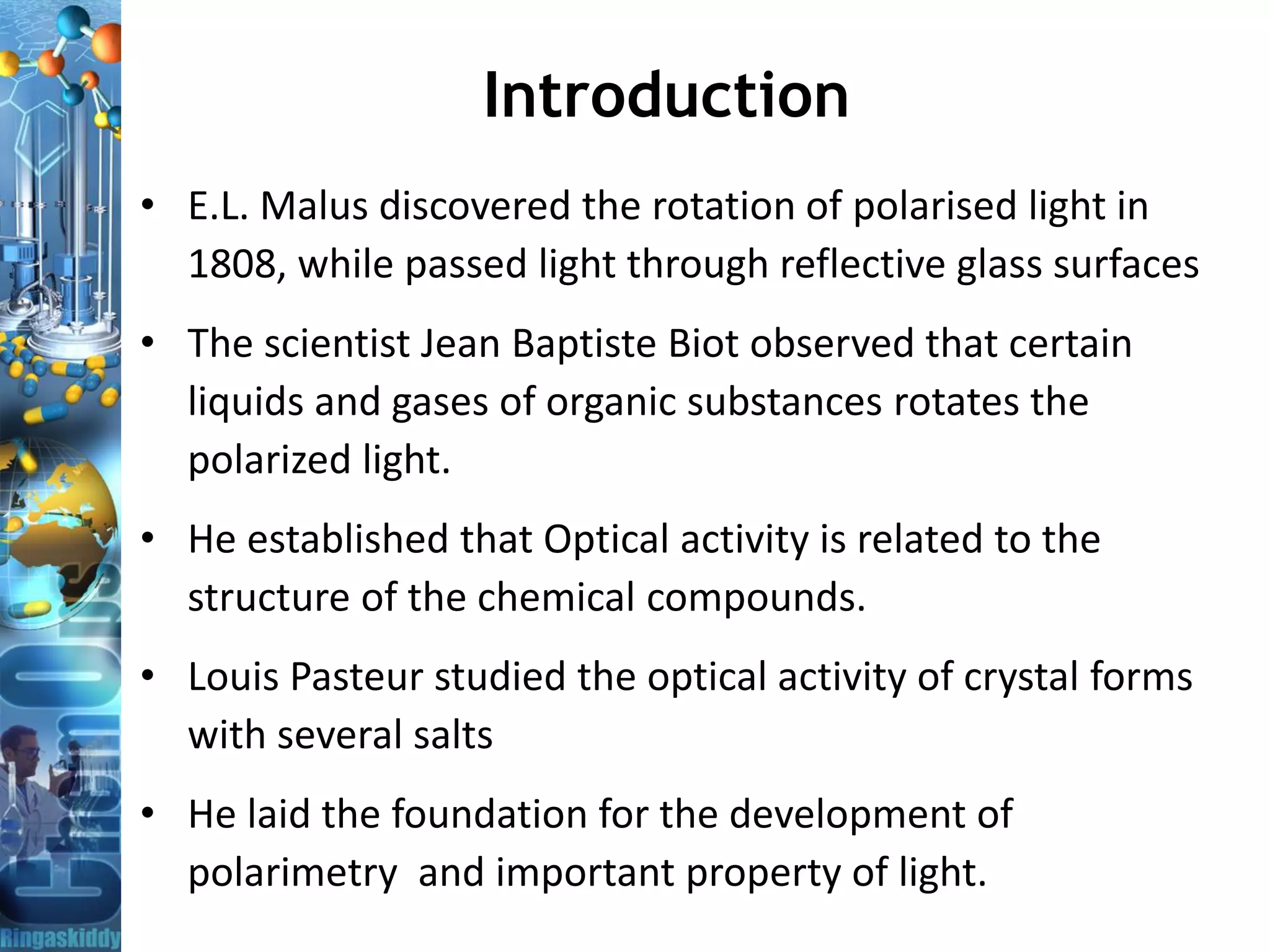
The document provides a comprehensive overview of optical rotation, polarimetry, and their applications in various industries, particularly pharmaceuticals. It discusses the principles of isomers, chiral molecules, and the role of polarimeters in measuring optical rotation, which is critical for characterizing the properties of optically active substances. Additionally, it emphasizes the importance of enantiomers in pharmacology, as their differing effects on biological systems can have significant implications for drug safety and efficacy.

![Stereoisomers
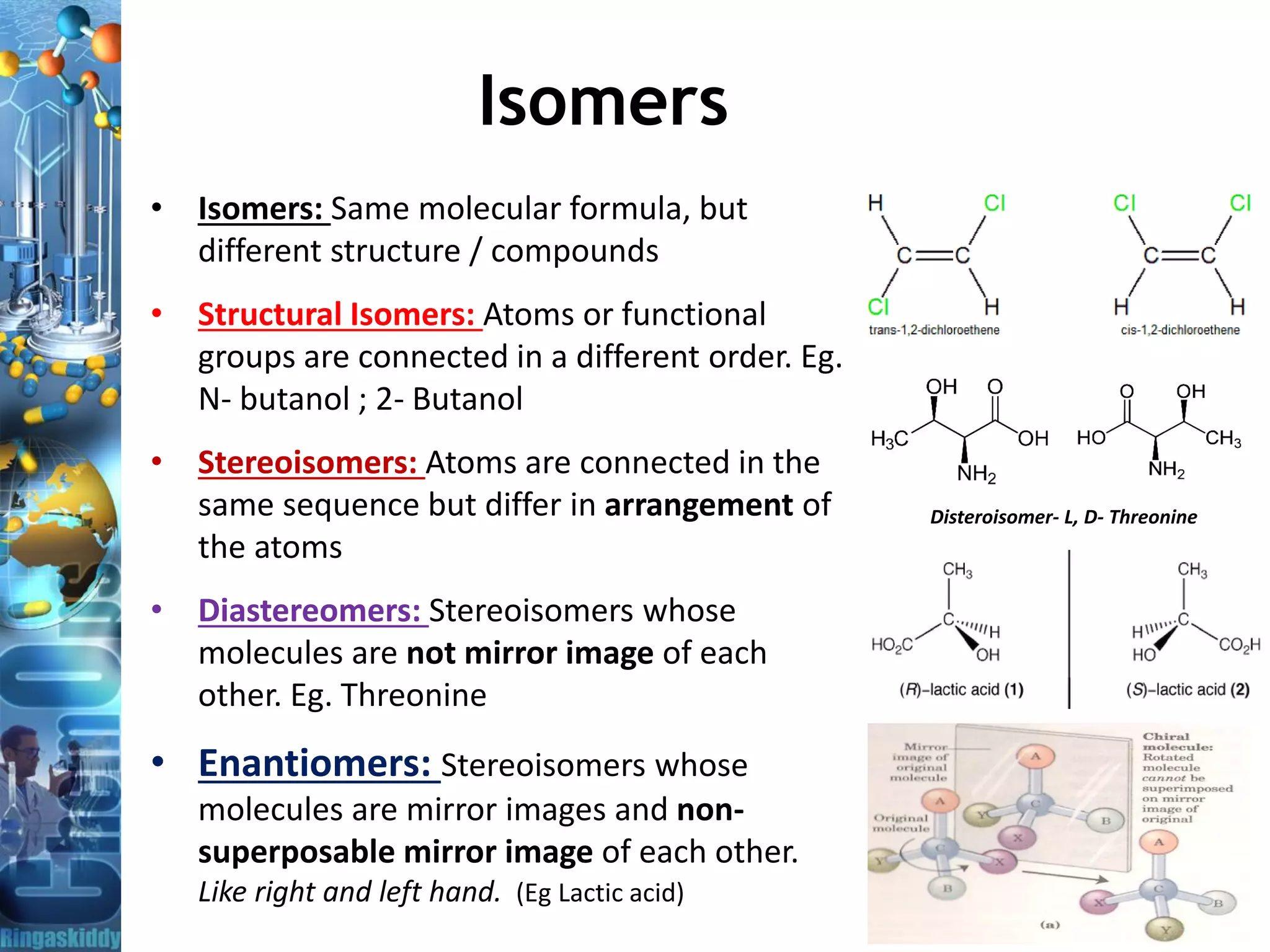
• Stereoisomers have chiral center(s). Two molecules are described
as stereoisomers if they are made of the same atoms connected in
the same sequence, but the atoms are positioned differently in space
( Spatial arrangement).
• Diastereomers have different physical properties like boiling
points, melting points, solubility.. etc
• Enantiomers: Physical properties like melting point, boiling point,
refractive index, etc. are identical and differ in a optical activity.
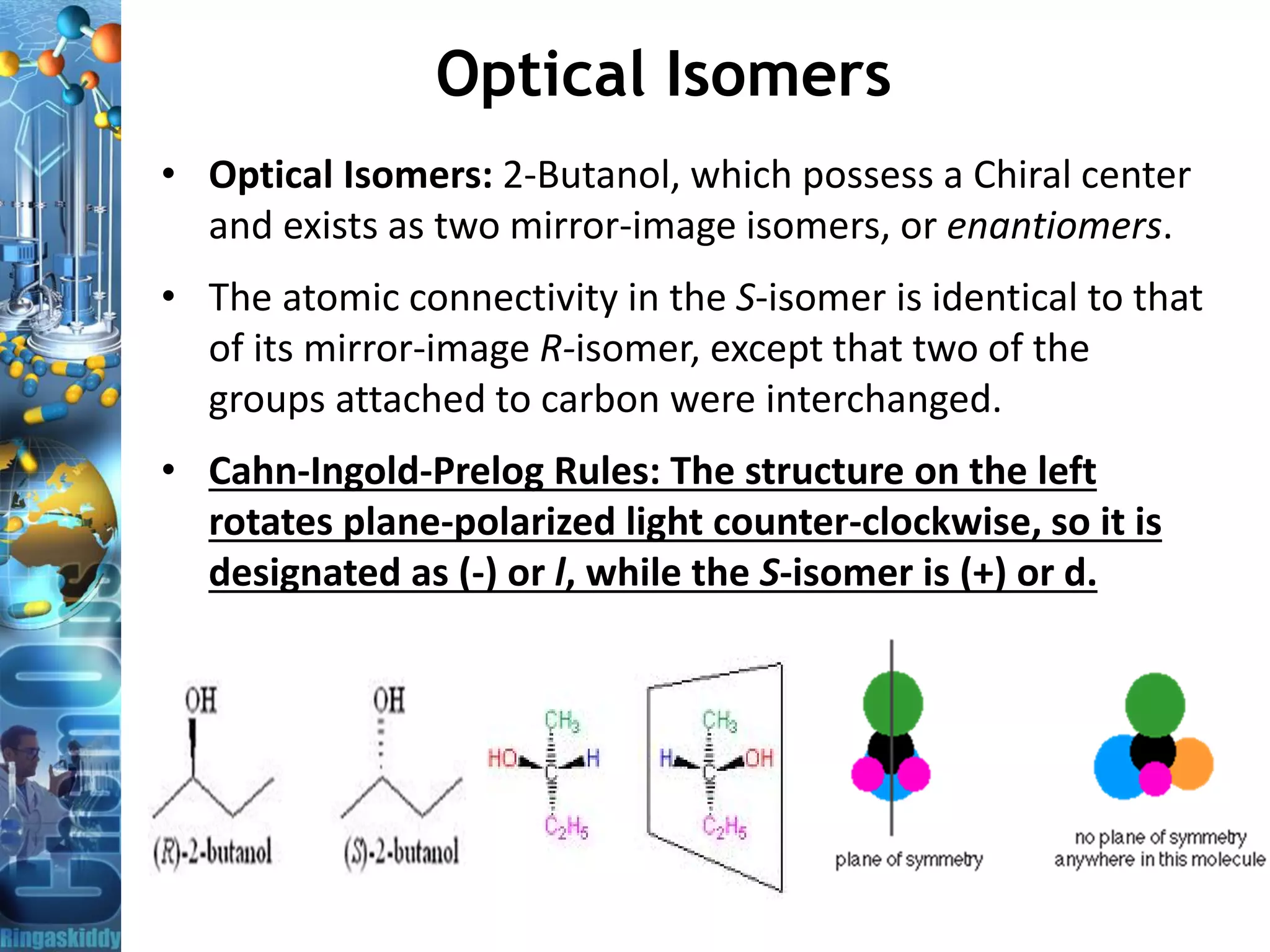
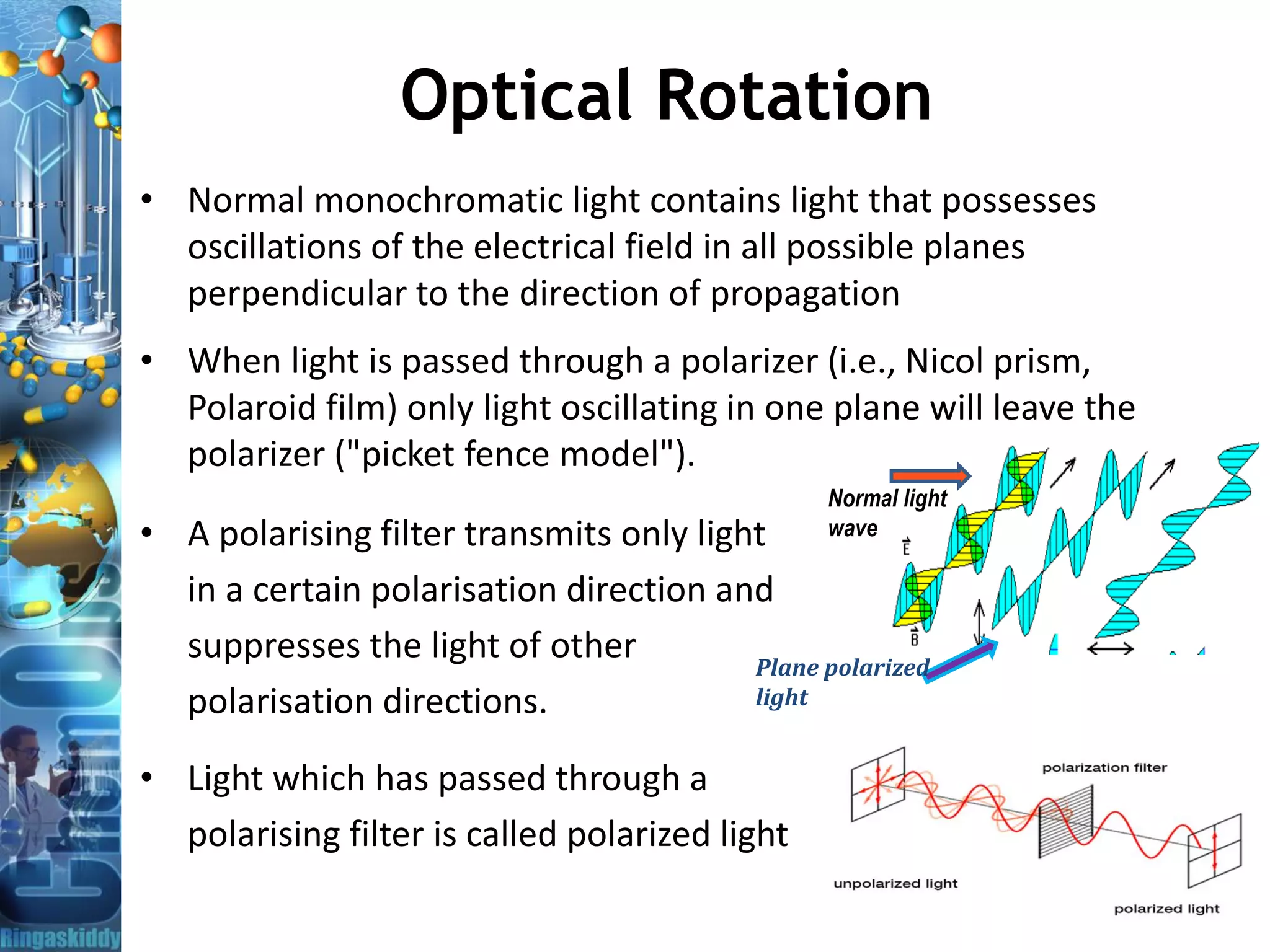
– Optical activity: Chiral molecules rotate the plane of polarized light as it
passes through solution to clockwise and counter-clockwise.
• Enantiomers turn the polarization plane in opposite directions.
• The angle by which the polarization plane is rotated is called optical
rotation. It is measured in degrees Arc [°] using Polarimeter.](https://image.slidesharecdn.com/opticalrotationandpolarimeter-bydr-210419110219/75/Optical-Rotation-and-Polarimeter-by-Dr-A-Amsavel-5-2048.jpg)




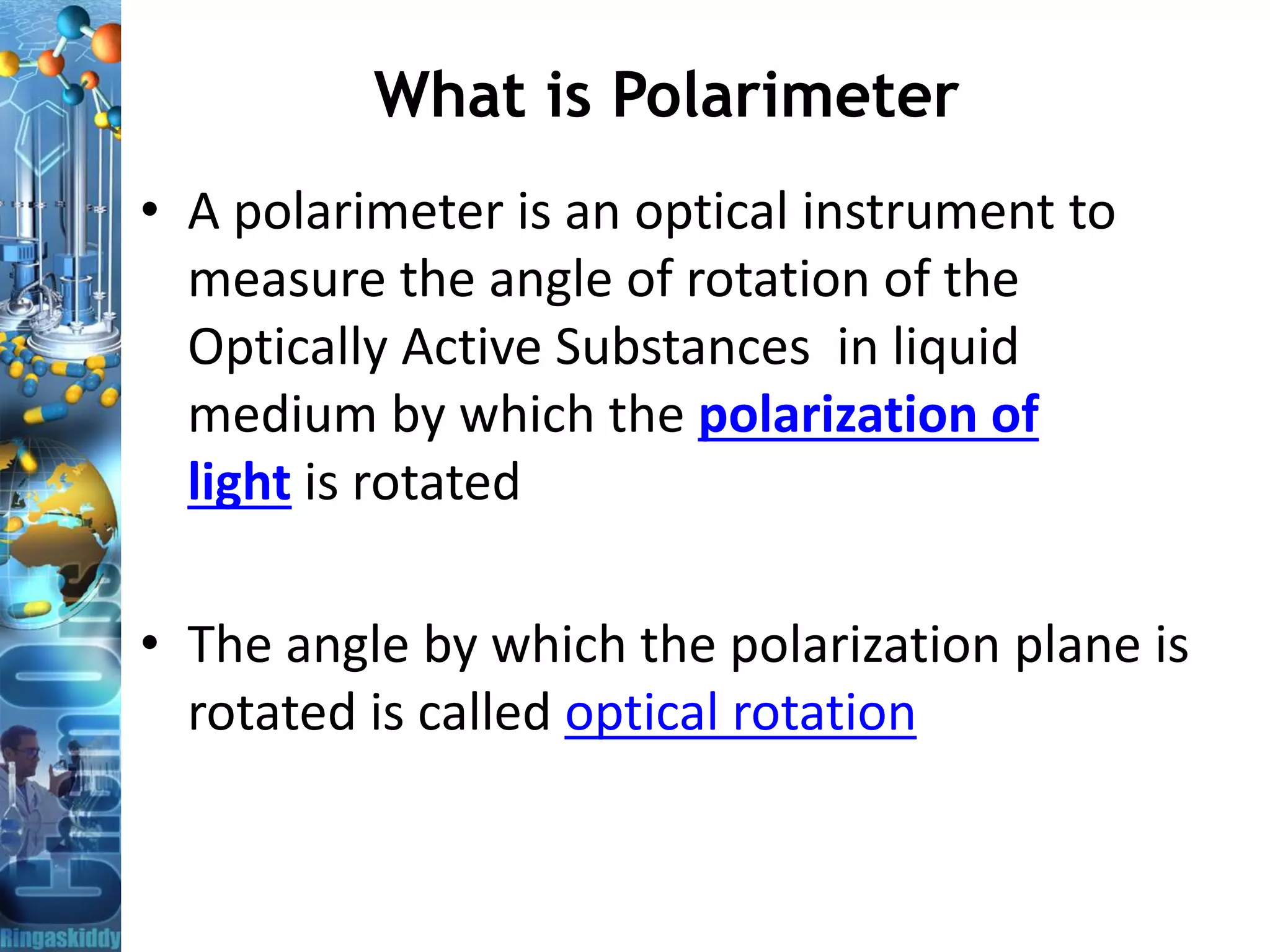
![Specific Optical Rotation
Optical rotation is determined using a polarimeters
The general equation for Specific Optical Rotation;
[α] = specific rotation at wavelength λ
T = temperature #
a = Observed rotation in degrees (°)
l = path length (dm)
C = concentration of the analyte (g/100 mL)
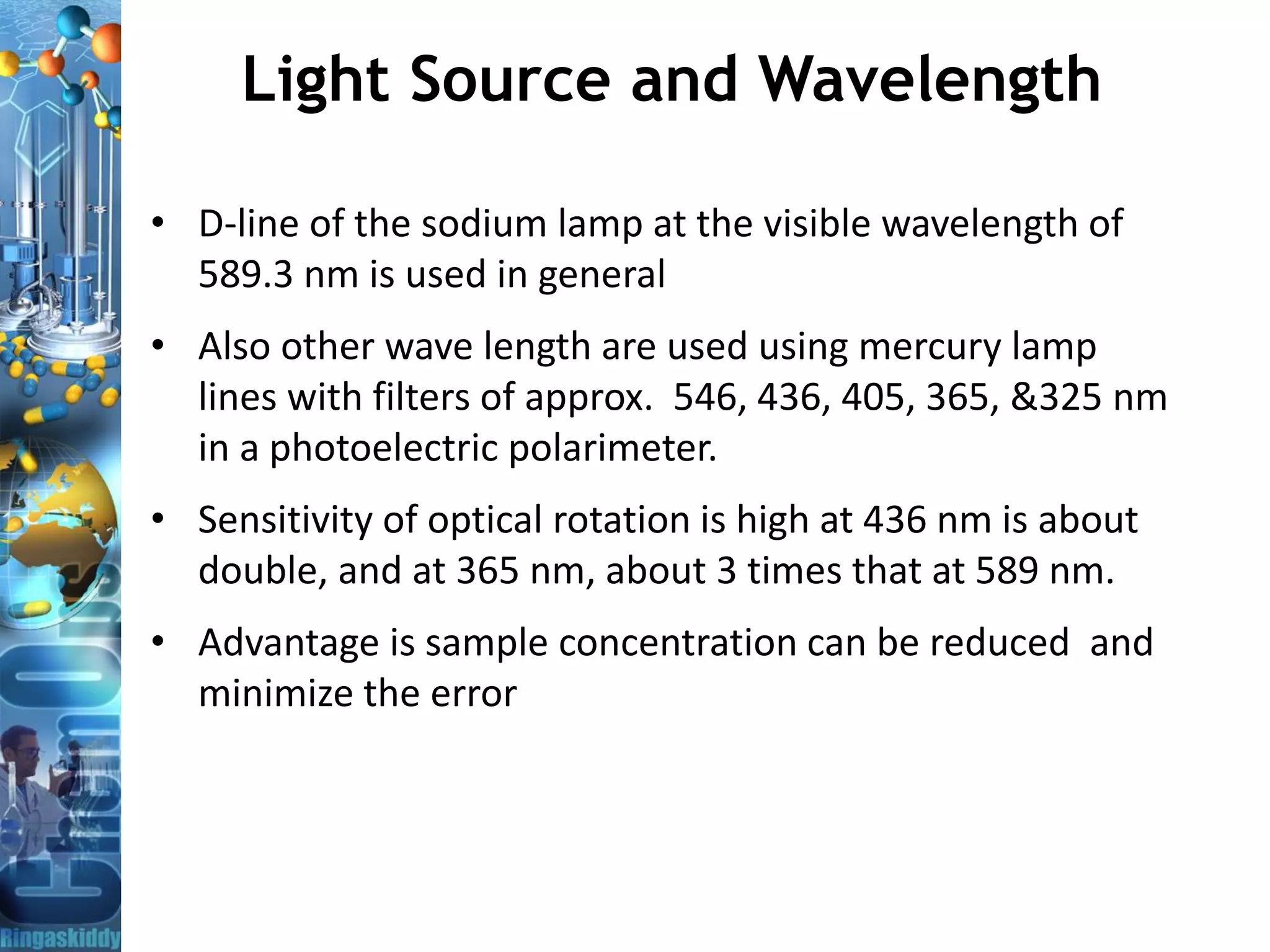
D-line of the sodium lamp at the visible
wavelength of 589.3 nm
As per USP optical rotation are made in a 1.0-dm tube at 589 nm at 25°C ± 0.5 °C
EP/BP -optical rotation are made in a 1.0-dm tube at 589 nm at 20°C ± 0.5 °C
Unit for SOR: There is no unit, but it
is understood as
degree millilitres per decimetre
gram [(°)·ml·dm− 1·g− 1]](https://image.slidesharecdn.com/opticalrotationandpolarimeter-bydr-210419110219/75/Optical-Rotation-and-Polarimeter-by-Dr-A-Amsavel-13-2048.jpg)
![Factors Affect The Optical Rotation
The angle of rotation may have influenced by:
1. Concentration of the sample, but SOR will not change, since
concentration is consider in the calculation.
2. Wavelength of light passing through the sample. Angle of
rotation and wavelength tend to be inversely proportional
3. Temperature of the sample (generally the two are directly
proportional)
4. Path length of the sample cell
5. Solvent used for solution preparation #
6. Operation: Filling conditions (bubbles, temperature and
concentration gradients)
# Example, Sample of natural (R,R)-tartaric acid, a change in solvent from water to a 1:1 mixture of
ethanol/chlorobenzene at same concentration, Change of [α]D
20 from +14.4 to –8.09.](https://image.slidesharecdn.com/opticalrotationandpolarimeter-bydr-210419110219/75/Optical-Rotation-and-Polarimeter-by-Dr-A-Amsavel-14-2048.jpg)
![Factors Influence the SOR
• The specific rotation is a material constant. It is the optical rotation for
a “given number” of optically active molecules in the light’s way
through the sample.
• Concentration of the optically active substance, c [g/mL]: the higher,
the more molecules
• Length of sample cell, l [mm]: the longer, the more molecules along
the way
• Temperature, T [°C]: influences density via thermal expansion and may
cause changes in molecular structure with effects on the optical
rotation
• Solvent: Changes the optical rotation value
• Another influencing parameter on the measured optical rotation is the
wavelength of the light, λ [nm].](https://image.slidesharecdn.com/opticalrotationandpolarimeter-bydr-210419110219/75/Optical-Rotation-and-Polarimeter-by-Dr-A-Amsavel-15-2048.jpg)