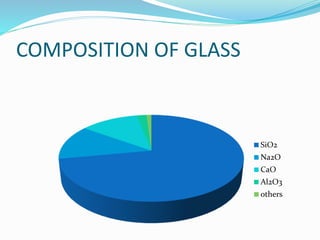
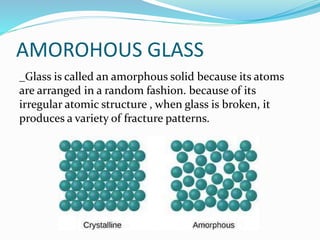
Glass is a non-crystalline solid that is often transparent and has many uses. The document discusses the history, composition, manufacturing process, types and uses of glass. It notes that the first true glass was made in Syria, and over time glass-making became popular in Egypt and was later used for stained glass and other purposes in buildings. The main components of glass are silica, sodium oxide, calcium oxide and aluminum oxide. Glass can be colored through the addition of metal ions and comes in types like fused glass, borosilicate glass, soda-lime glass and lead crystal glass.