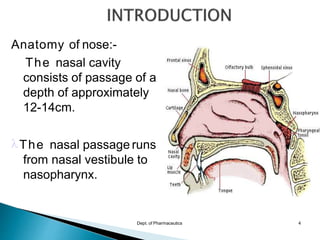
The document discusses drug delivery through the nasal and pulmonary routes. It begins by describing the anatomy of the nose and lungs. It then discusses various nasal and pulmonary drug delivery systems including liquid and solid dosage forms for nasal delivery as well as metered dose inhalers, dry powder inhalers, and nebulizers for pulmonary delivery. Finally, it outlines some advantages of these routes such as rapid drug absorption, avoidance of first-pass metabolism, and lower dosing requirements.