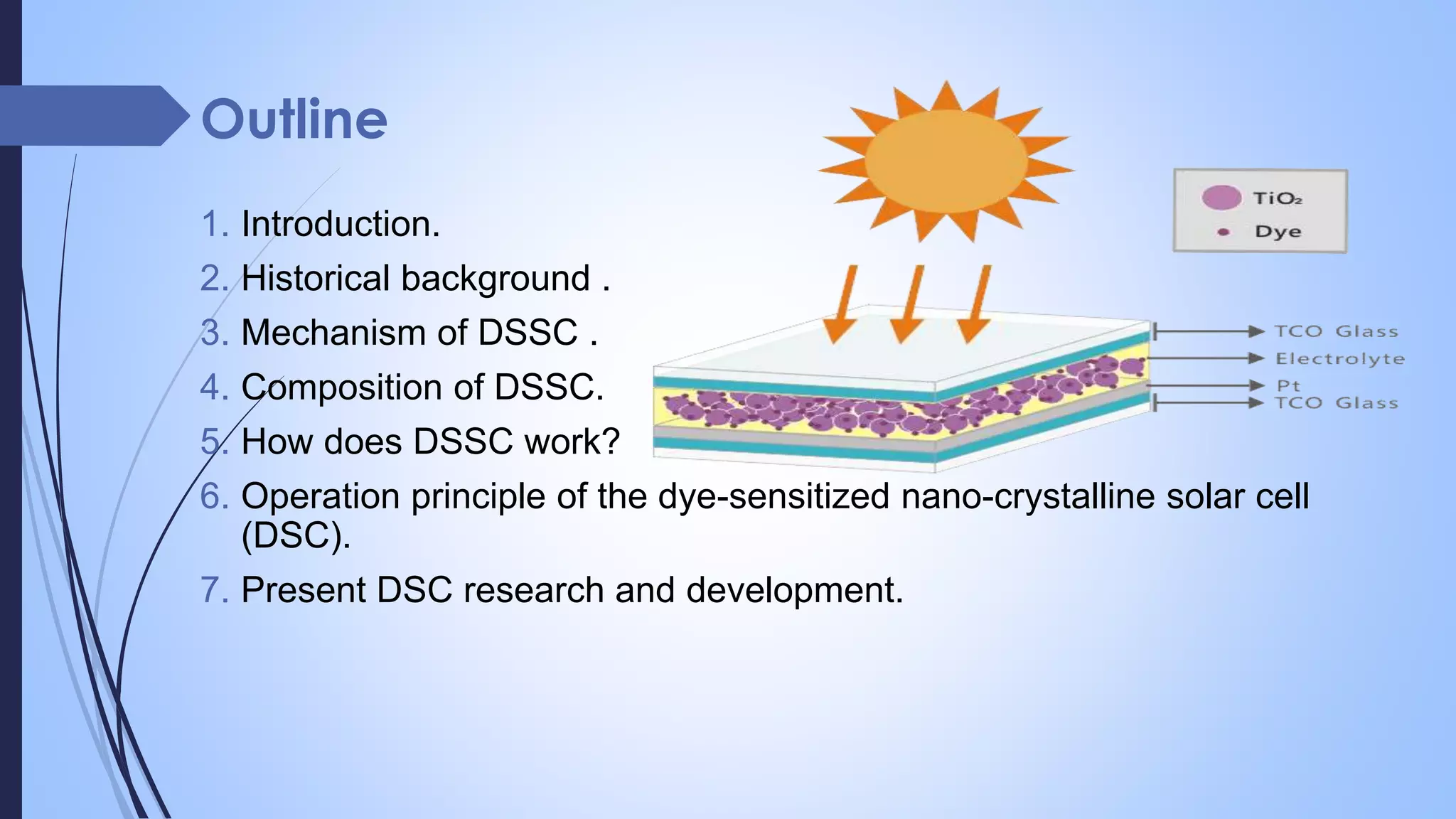
Dye-sensitized solar cells (DSSCs) are a type of solar cell that uses dye molecules to absorb sunlight and convert it to electrical energy. They were invented in 1991 by Brian O'Regan and Michael Grätzel. DSSCs consist of a photo-sensitized anode, an electrolyte containing a redox couple, and a cathode. When light is absorbed by the dye, electrons are injected into the conduction band of the semiconductor and transported through the external circuit to be collected at the cathode, while the dye is regenerated through the redox shuttle. DSSCs offer advantages such as low cost, flexibility in design, and the ability to work in low light conditions. Recent research aims to