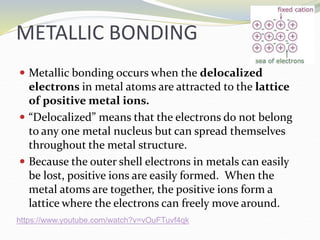
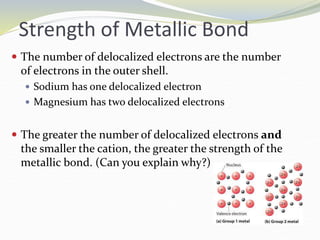
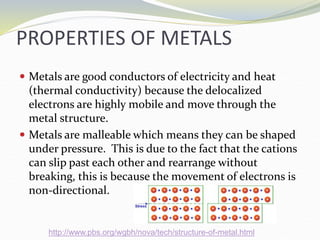
The document discusses metallic bonding, explaining that it involves a lattice of cations and delocalized electrons, leading to distinctive properties of metals like conductivity and malleability. Factors affecting metallic bond strength include the charge and size of cations, with stronger bonds resulting in higher melting points. Additionally, it covers alloys, which enhance properties compared to pure metals due to their structure and the non-directional nature of bonding.