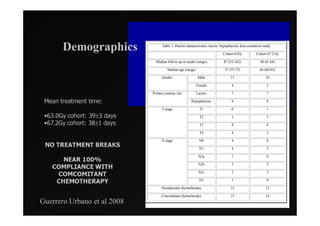
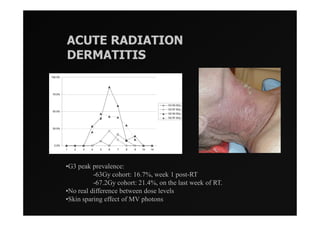
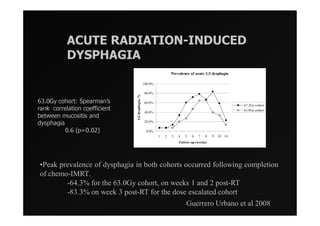
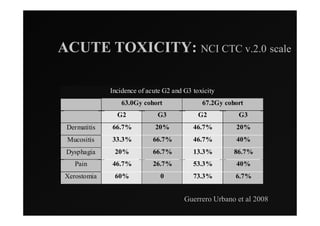
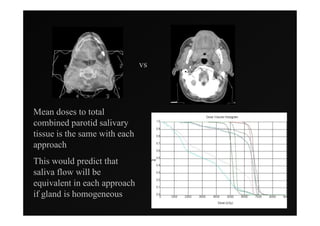
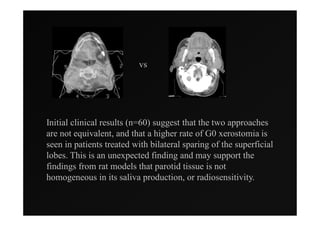
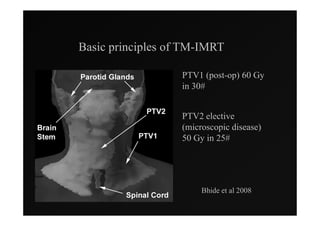
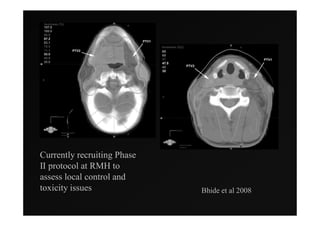
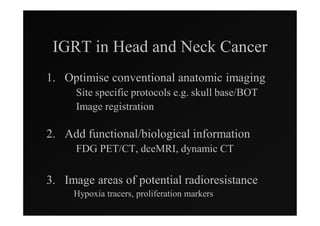
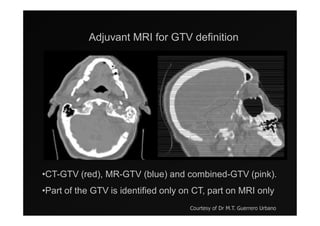
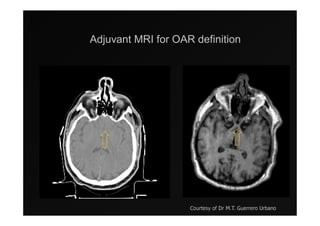
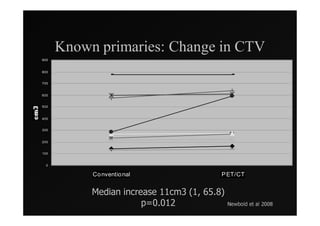
1) IMRT and IGRT techniques aim to improve outcomes for head and neck cancer patients by better targeting tumors and reducing toxicity to organs at risk.
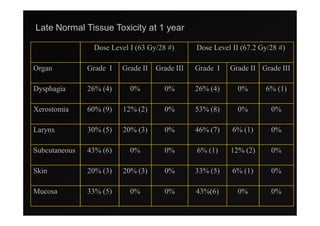
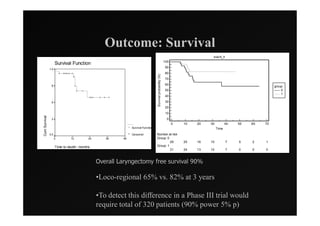
2) Early phase trials show dose escalated IMRT is feasible and improves local control for larynx and hypopharynx cancers compared to conventional radiotherapy.
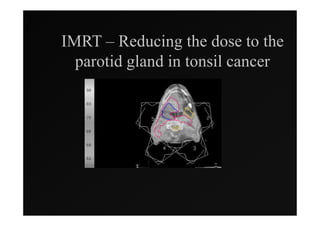
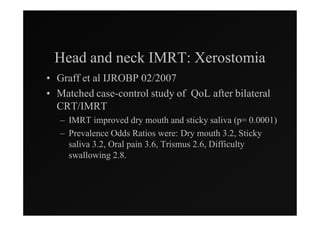
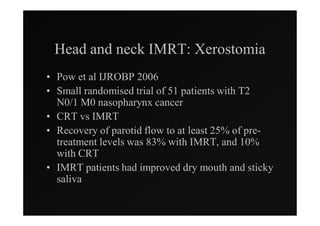
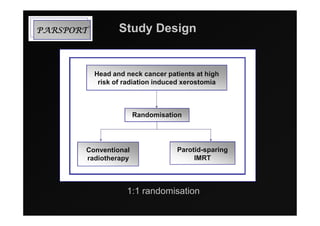
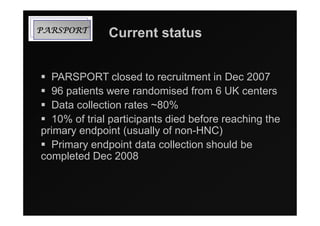
3) Ongoing randomized trials are investigating whether parotid gland-sparing IMRT reduces xerostomia compared to conventional radiotherapy for oropharynx cancers.
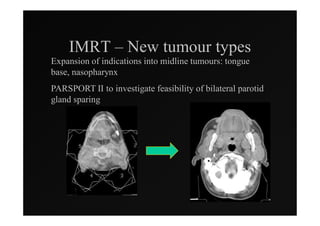
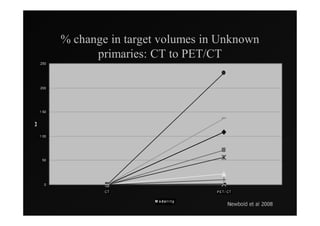
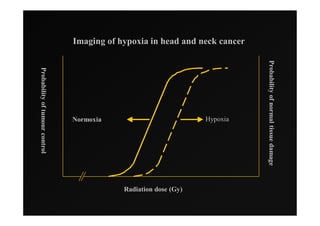
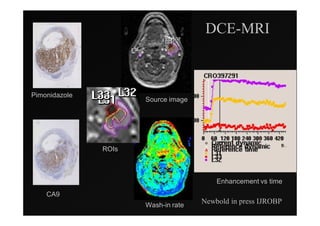
4) Novel applications of IMRT include its use for unknown primary cancers to potentially improve local control without high toxicity. Integration of imaging techniques with IMRT may further optimize treatment.