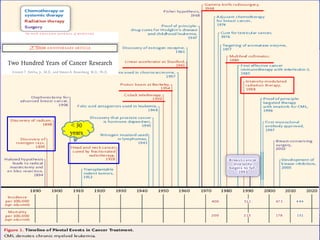
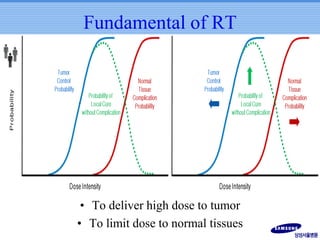
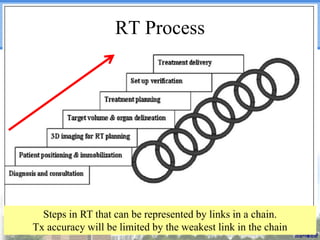
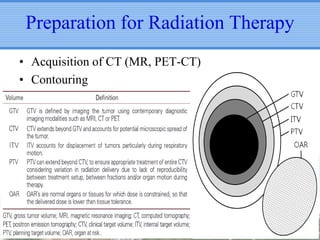
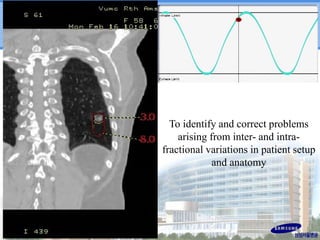
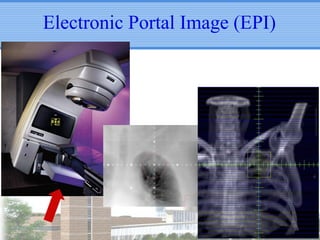
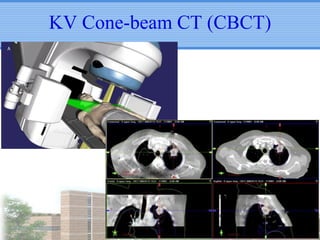
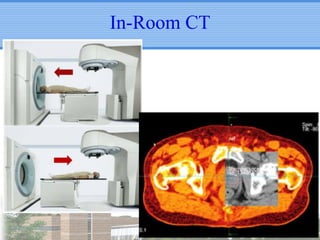
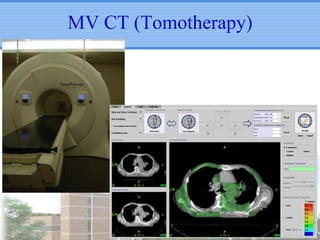
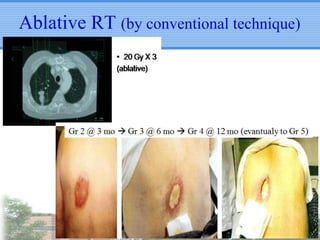
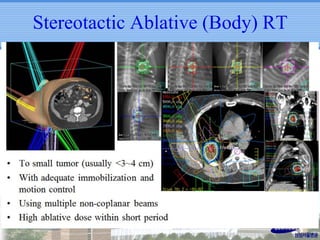
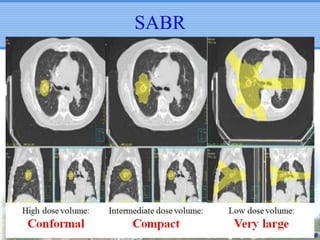
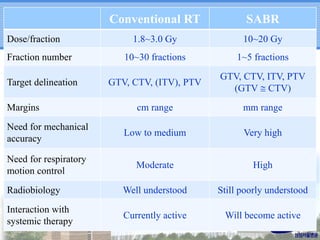
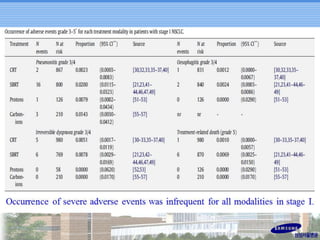
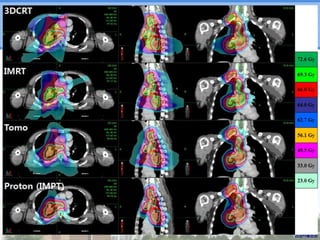
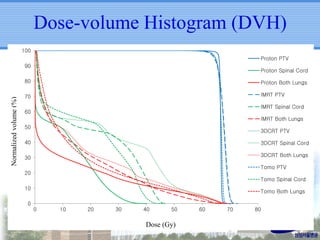
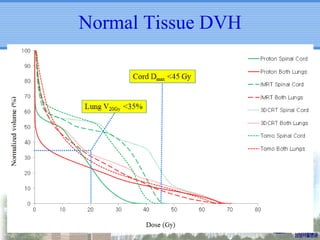
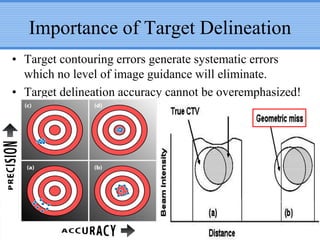
- Novel RT techniques such as SBRT, IMRT, IGRT and particle beam therapy can provide high local control rates for lung cancer with reduced toxicity compared to conventional RT.
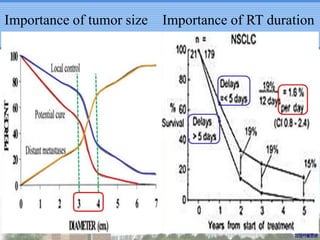
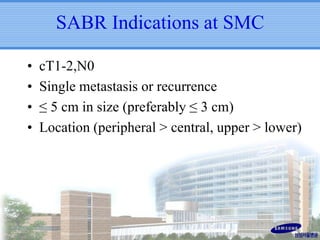
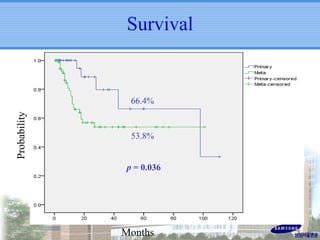
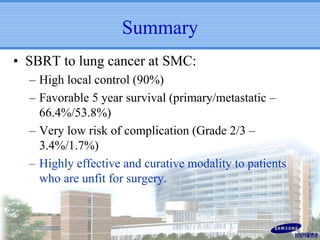
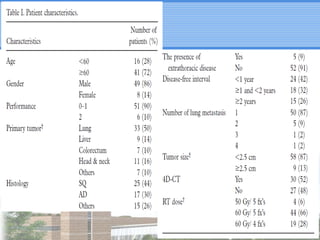
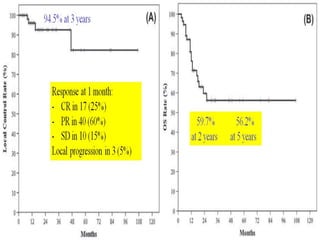
- SBRT achieved 90% local control and favorable 5-year survival for primary and metastatic lung cancers at SMC with very low complication risks.
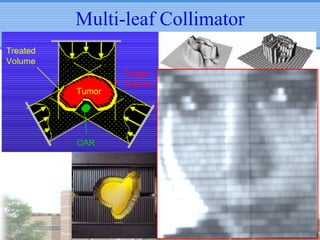
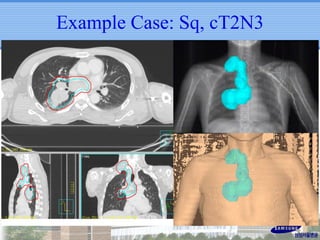
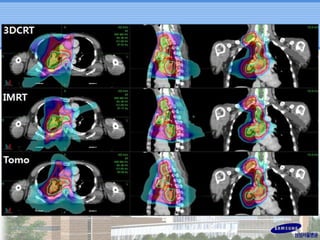
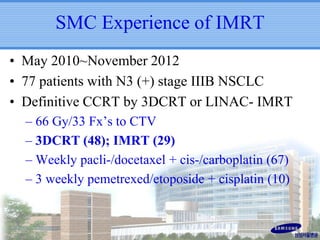
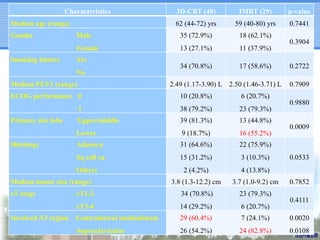
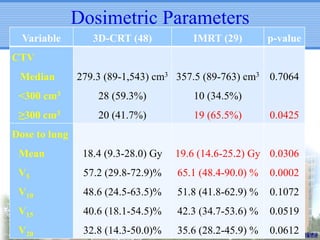
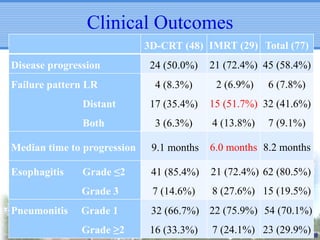
- IMRT may be beneficial for large or centrally-located tumors but further study is needed due to the study's retrospective nature and heterogeneous patient population.
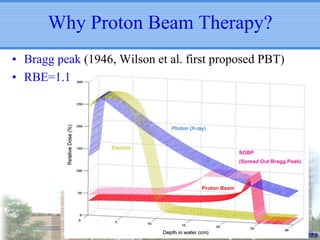
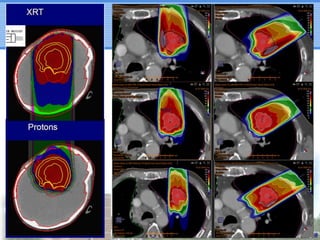
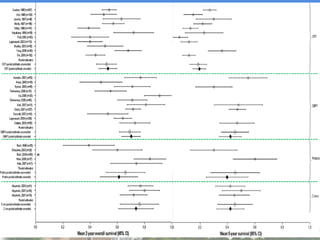
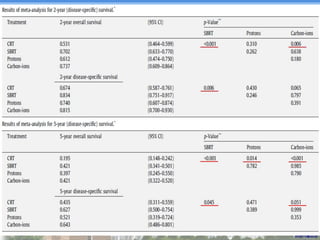
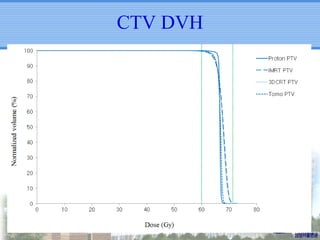
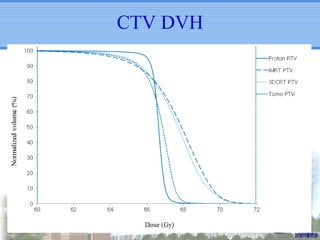
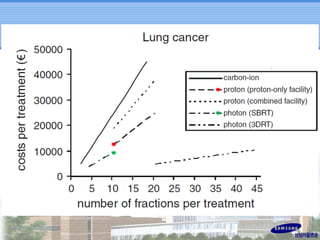
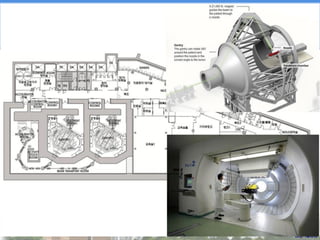
- Particle beam therapy, such as proton therapy, can further reduce dose to organs-at-risk compared to photon therapies and may allow dose escalation for improved outcomes, particularly for locally advanced lung cancers.