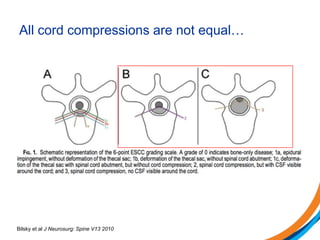
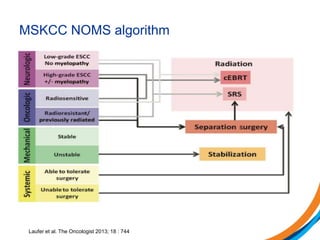
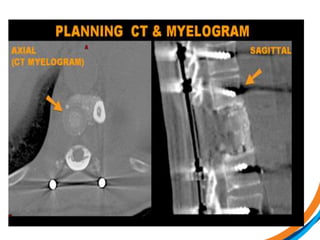
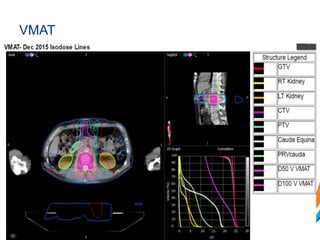
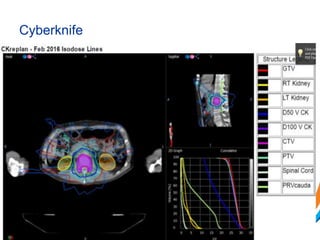
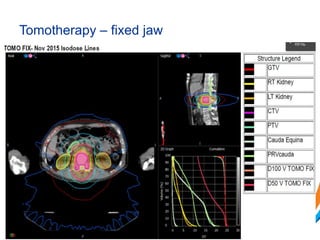
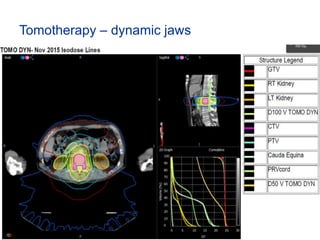
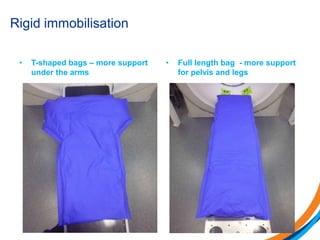
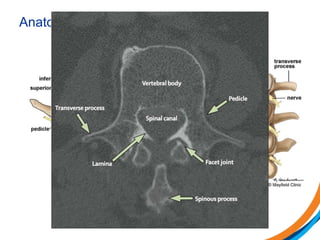
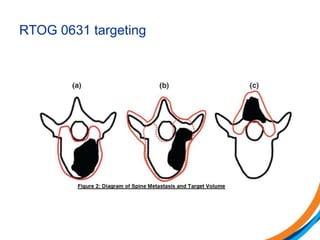
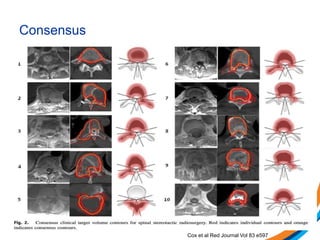
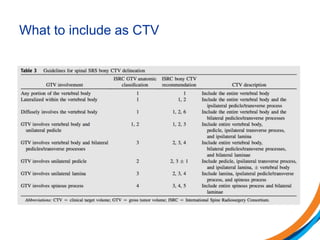
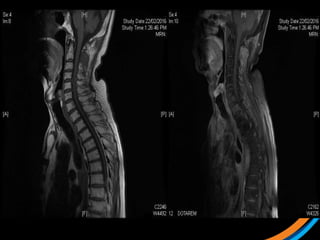
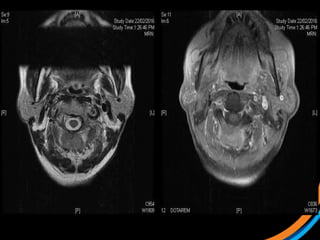
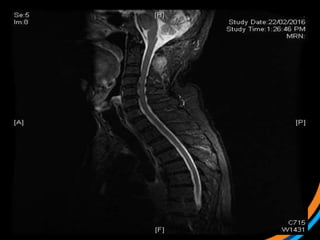
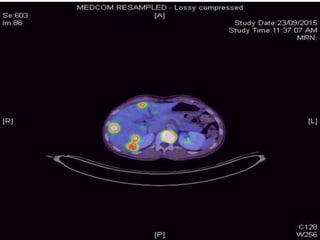
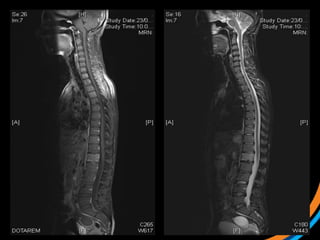
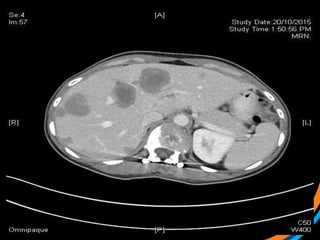
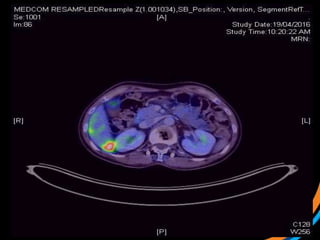
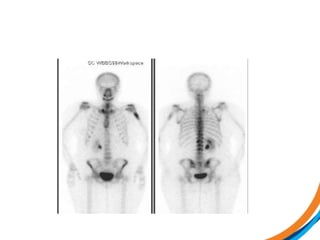
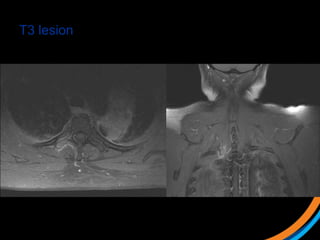
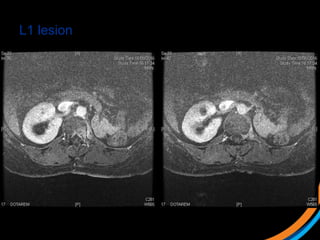
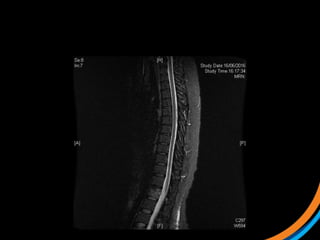
This document provides an overview and practical tips for spine stereotactic body radiation therapy (SBRT). It discusses patient selection criteria including good performance status and life expectancy. Required imaging includes MRI and CT to aid targeting of the gross tumor volume (GTV) and clinical target volume (CTV). Treatment planning considerations include dose selection of 24-35Gy in 3-5 fractions and organ at risk constraints. Delivery involves cone beam CT guidance to ensure accurate positioning. Case studies demonstrate targeting of spinal metastases from different primary cancers. The document emphasizes the importance of immobilization, image guidance and multidisciplinary care for safe and effective spine SBRT.