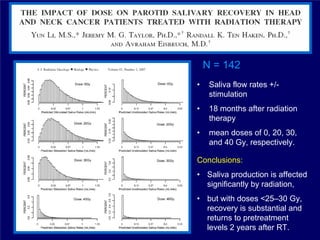
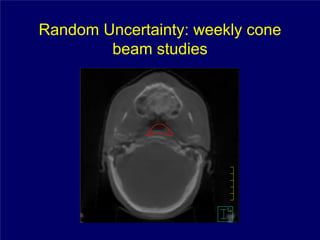
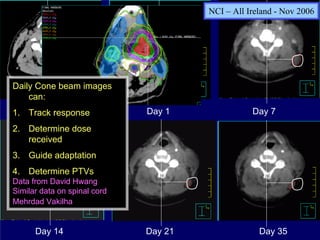
1) The document discusses optimal practice in radiation treatment for head and neck cancer in the 21st century, focusing on balancing treatment targets and sparing normal tissues using available technology and expertise.
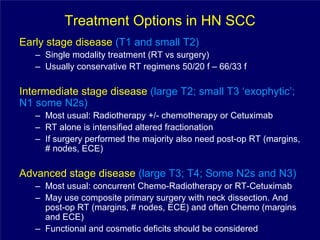
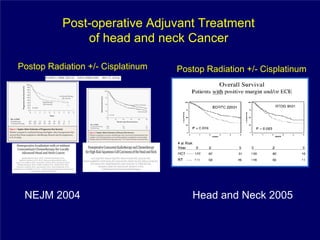
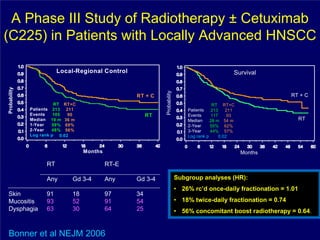
2) It reviews treatment options and approaches for different stages of head and neck cancer, highlighting evidence that altered fractionation and chemoradiation can improve outcomes over standard radiation alone.
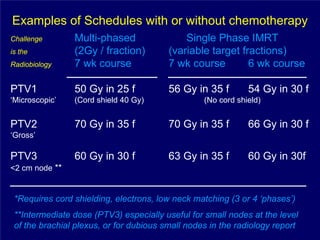
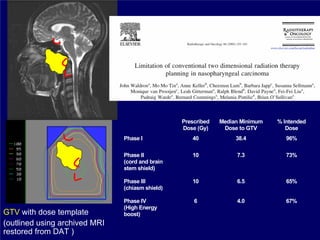
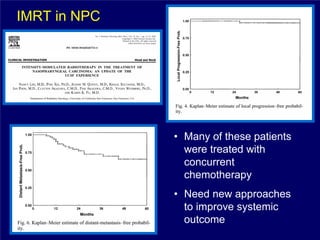
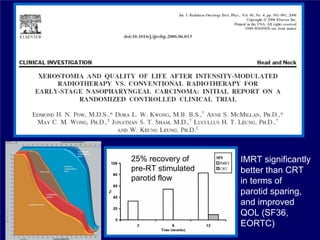
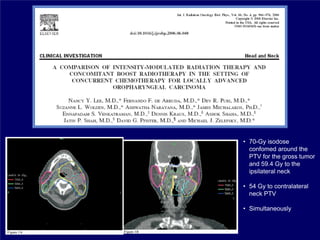
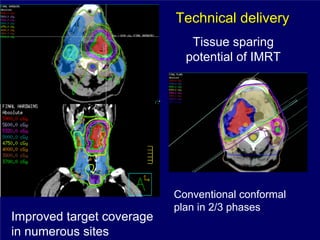
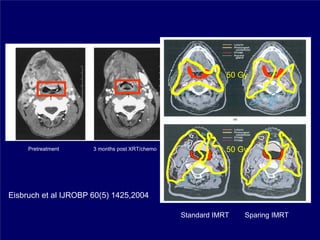
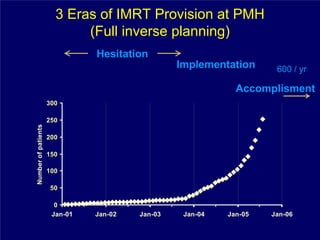
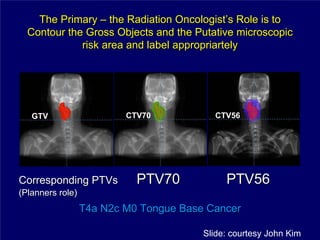
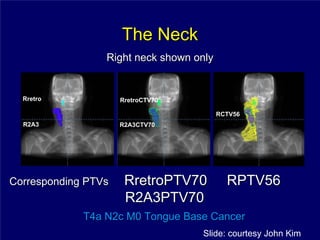
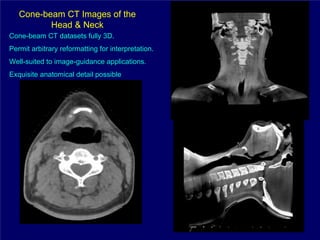
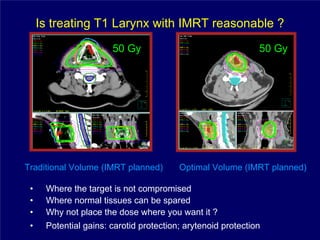
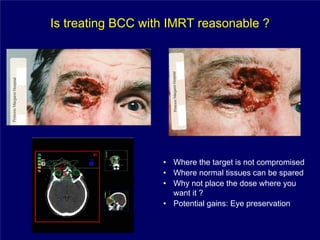
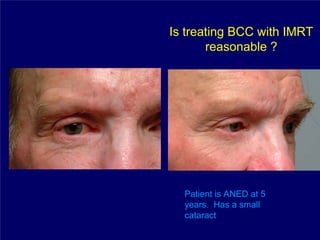
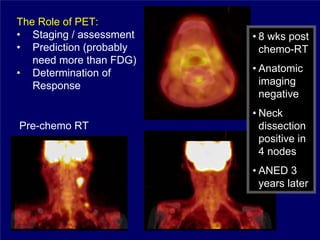
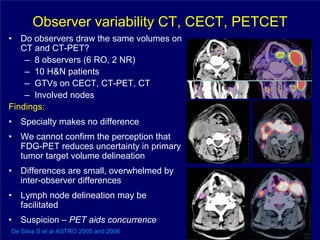
3) Challenges of implementing intensity-modulated radiation therapy (IMRT) for head and neck cancer are discussed, as well as examples of how IMRT can improve target coverage and tissue sparing compared to conventional techniques.