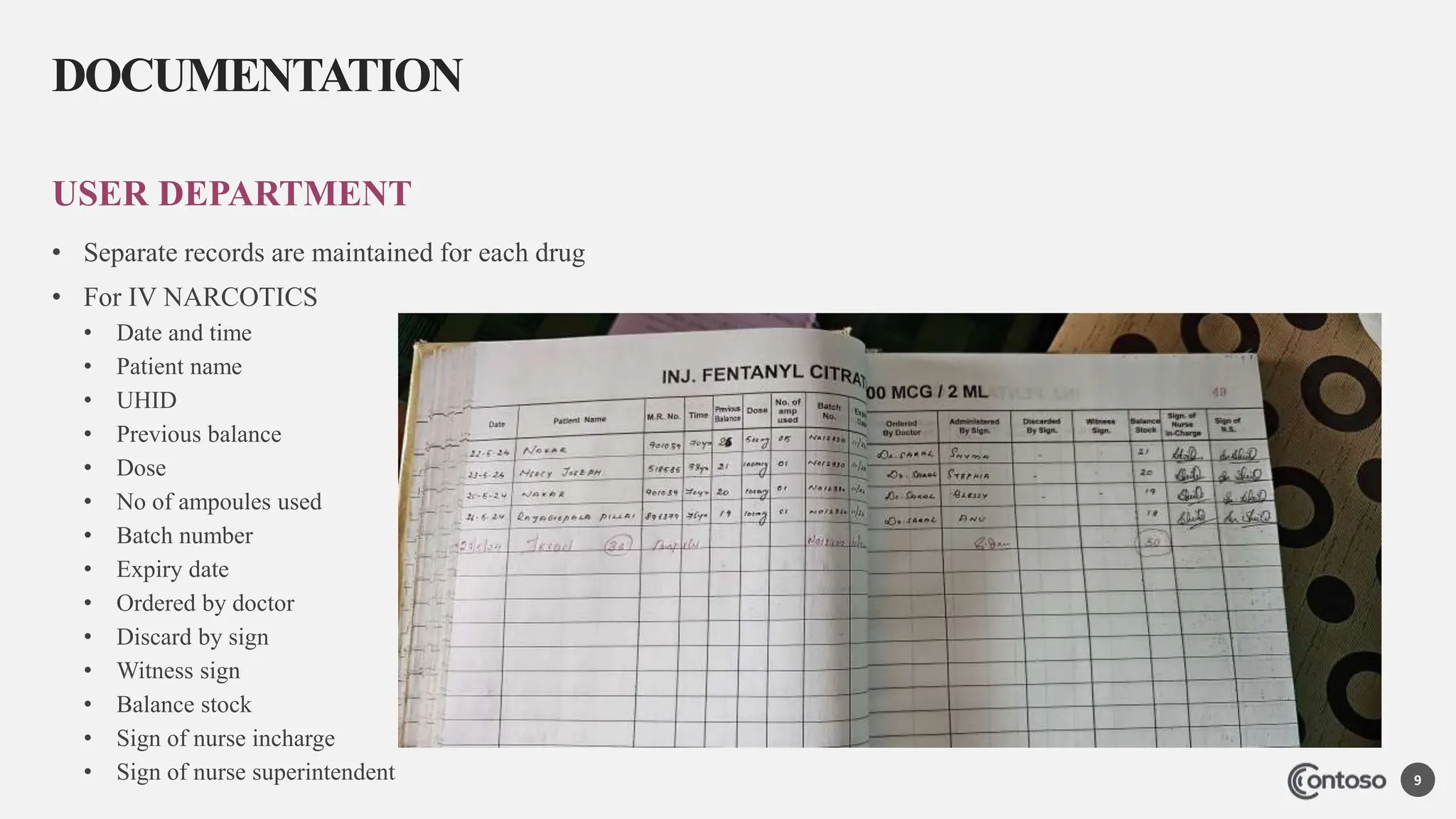
The document outlines the policy and procedures for the safe and rational use of narcotics and psychotropic substances in a clinical setting. It specifies the responsibilities of healthcare personnel, proper storage under double lock, and meticulous record-keeping. Additionally, it mandates the maintenance of documentation for all narcotic transactions for a period of two years and includes instructions for handling prescriptions and expired drugs.