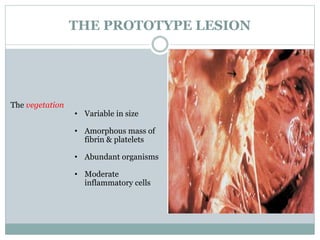
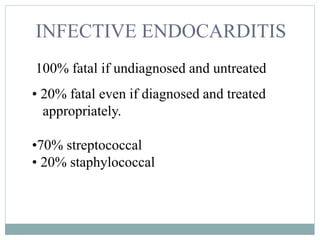
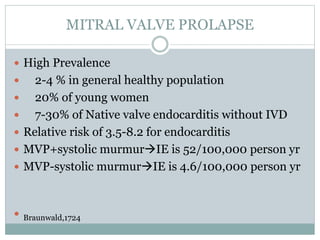
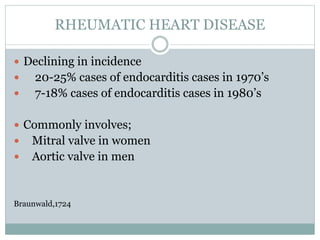
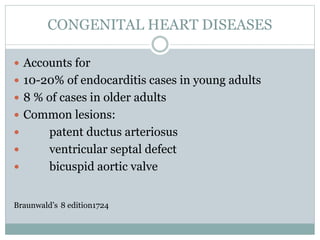
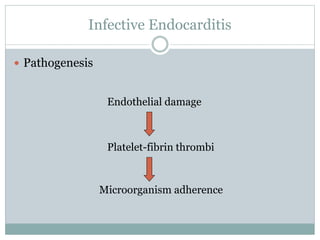
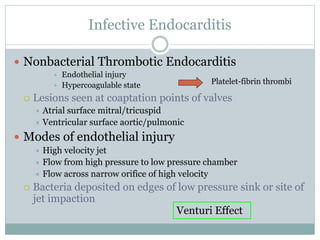
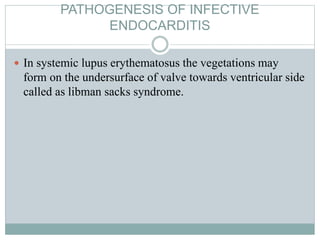
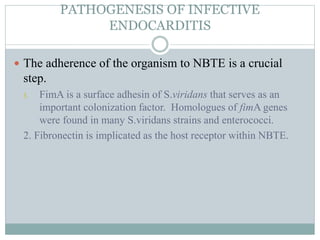
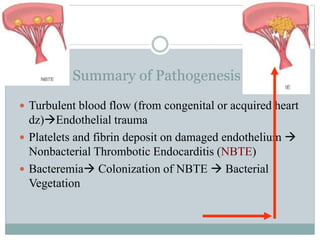
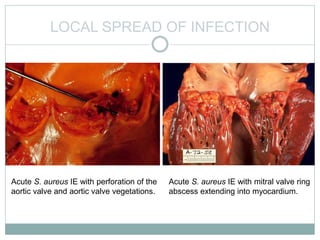
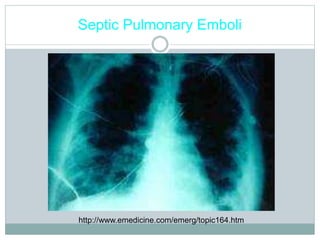
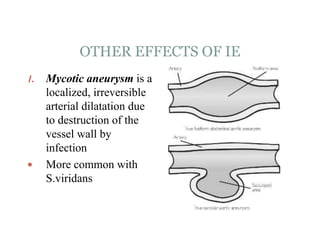
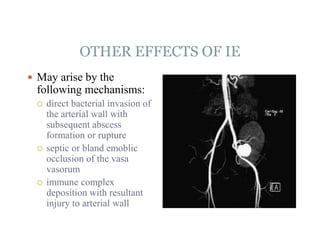
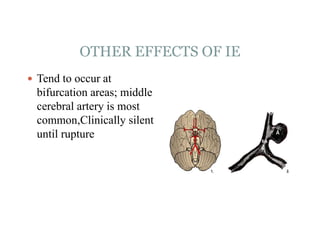
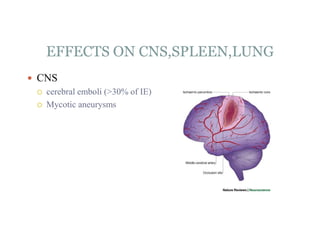
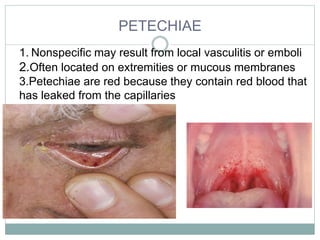
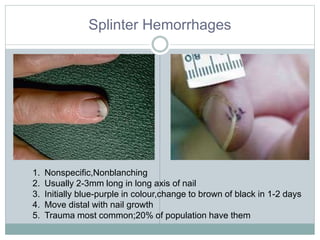
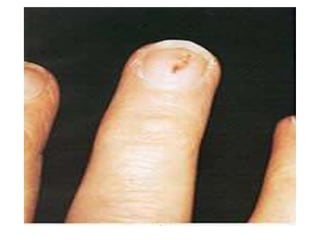
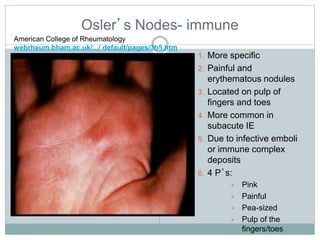
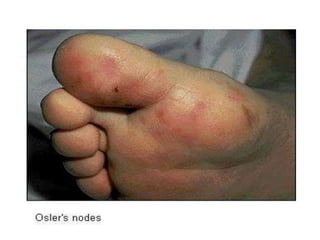
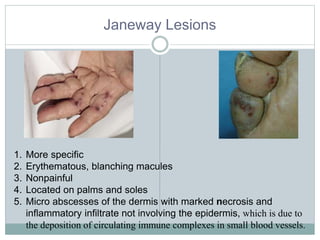
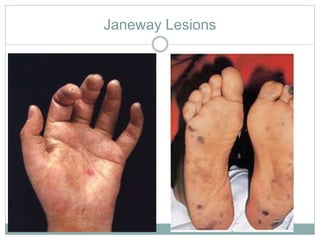
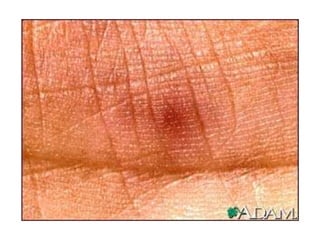
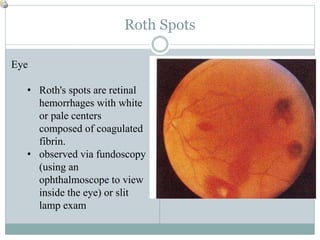
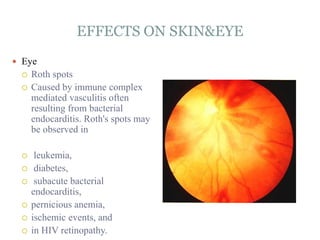
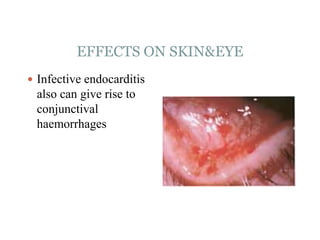
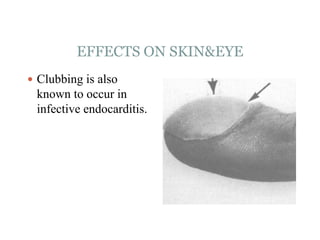
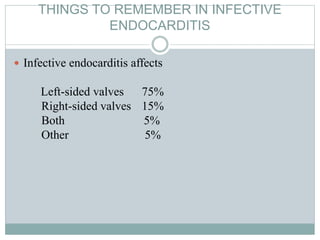
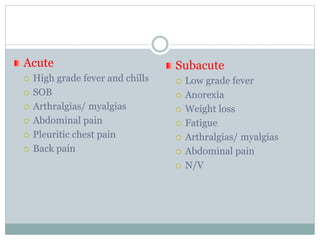
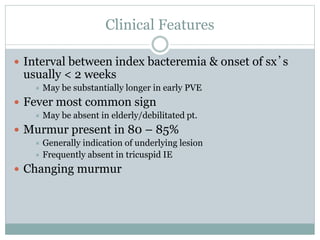
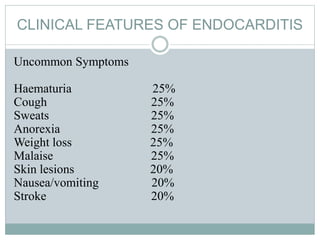
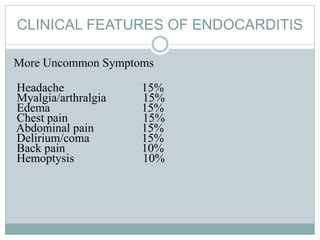
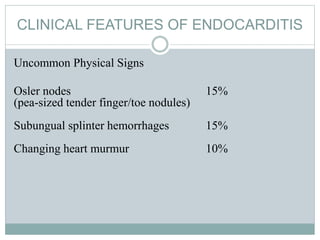
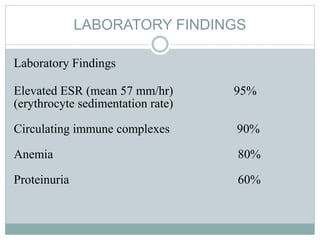
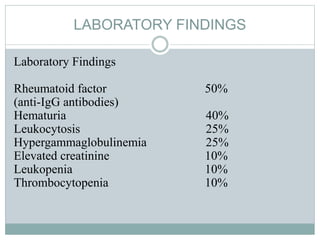
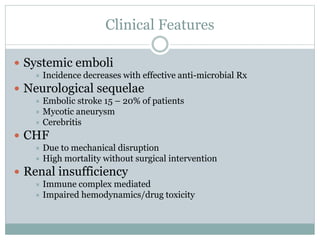
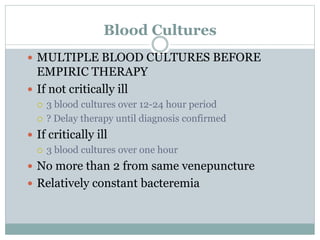
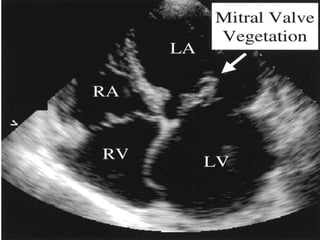
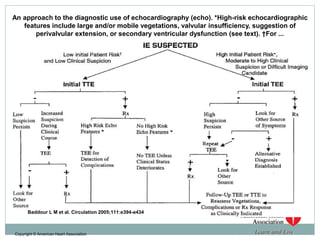
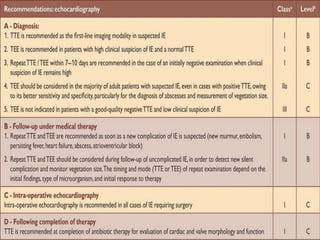
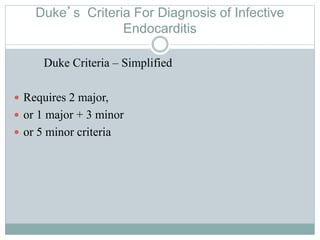
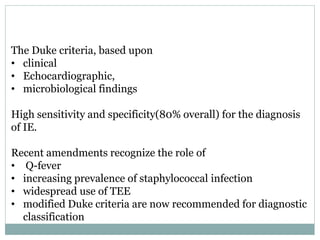
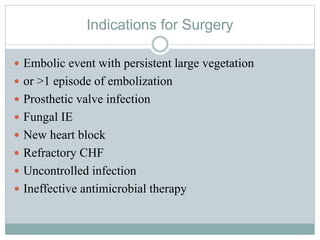
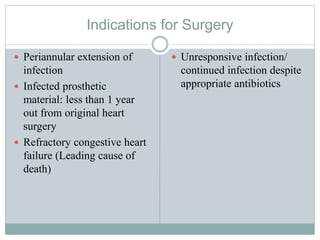
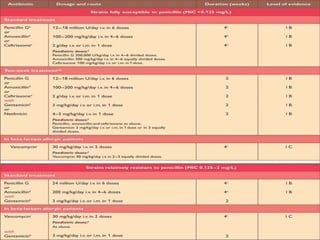
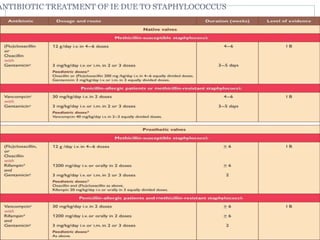
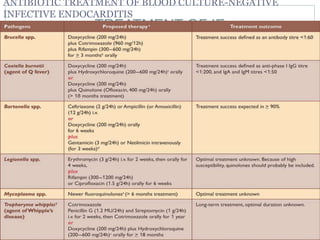
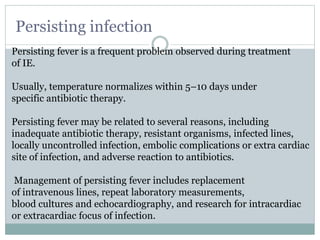
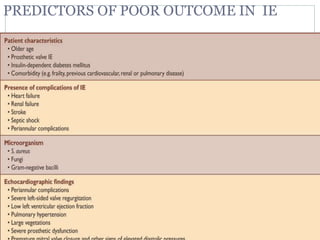
Infective endocarditis is a microbial infection of the heart valves or endocardium. It typically involves the valves and can be caused by many pathogens. The most common causes are streptococci, staphylococci, and enterococci. Untreated infective endocarditis has a high fatality rate. The pathogenesis involves endothelial damage, platelet-fibrin deposition forming nonbacterial thrombotic endocarditis (NBTE), and microbial colonization of the NBTE resulting in bacterial vegetations. Local effects include valvular damage, abscesses, fistulae, and conduction abnormalities. Distant effects occur via septic emboli that can lodge in organs like the brain, lungs,