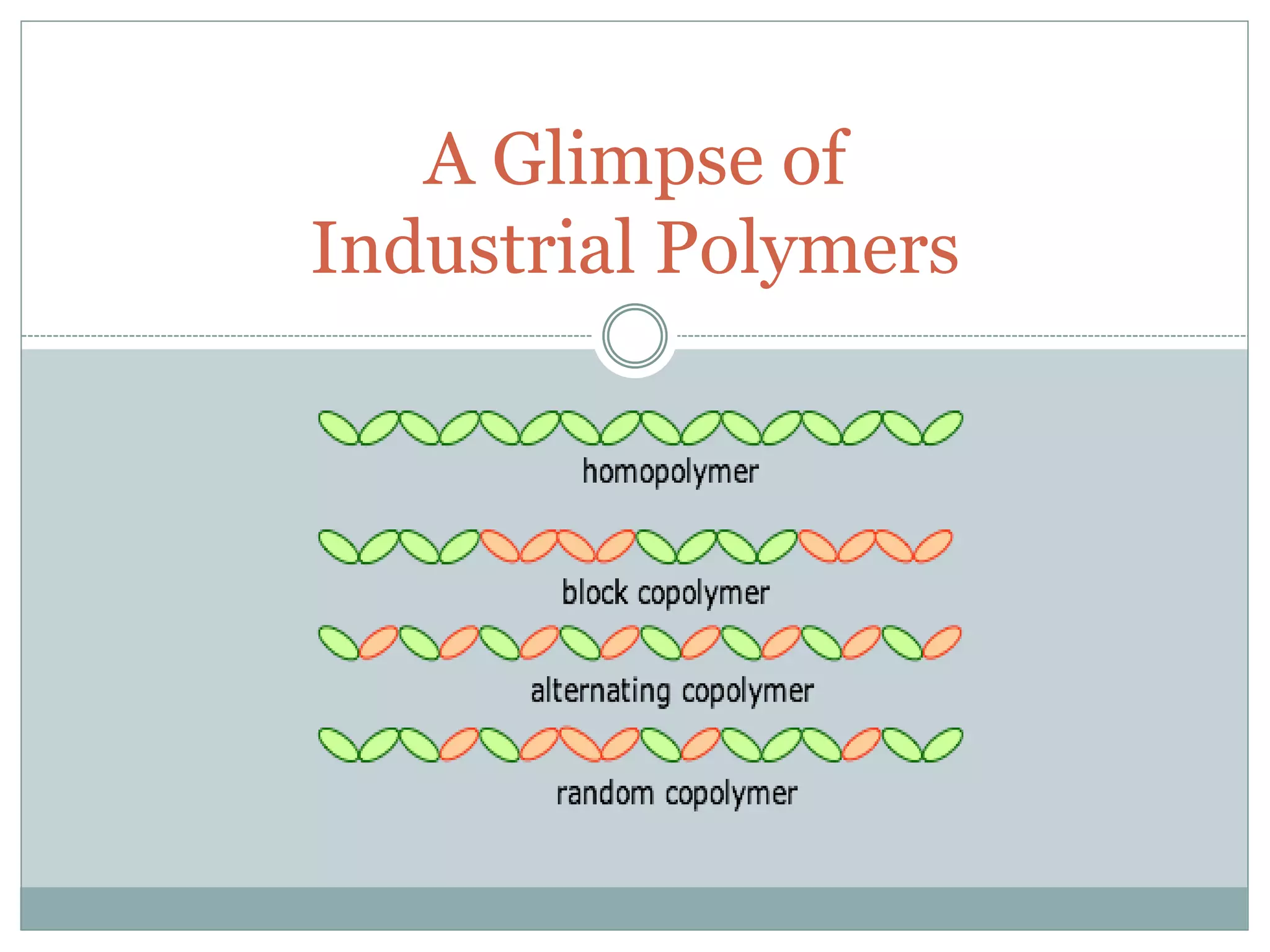
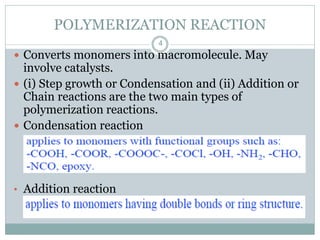
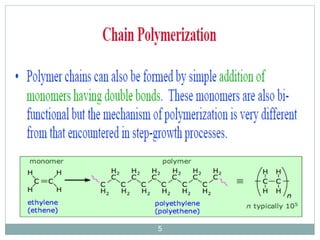
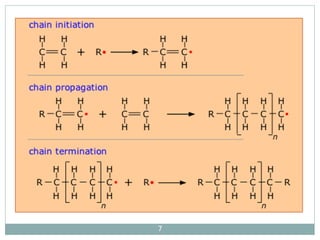
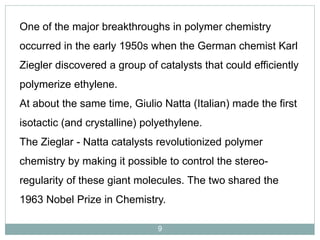
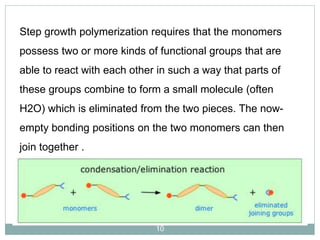
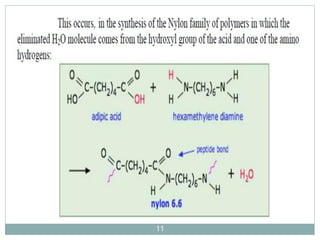
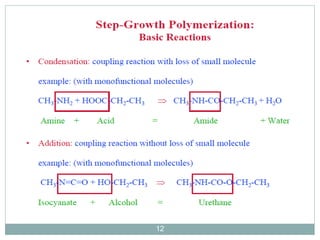
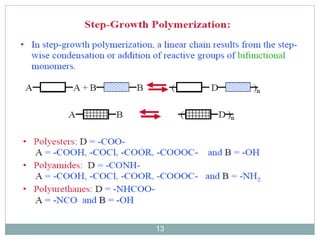
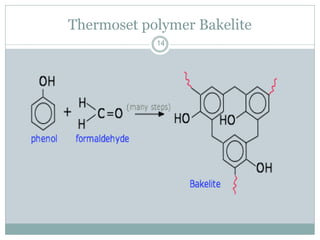
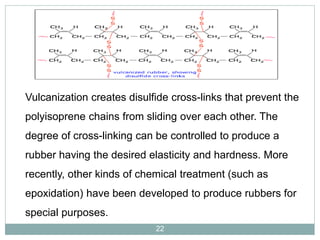
The document discusses industrial polymers, including definitions, types of polymerization, and examples of natural and synthetic polymers. It highlights the significance of breakthroughs in polymer chemistry, such as the Ziegler-Natta catalysts, and provides information on various synthetic materials like rubber, fibers, and biodegradable plastics. Additionally, it touches on the environmental concerns associated with non-degradable plastics and the growing interest in biodegradable alternatives.