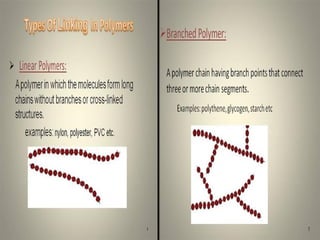
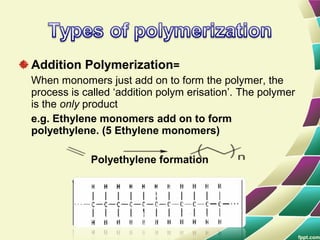
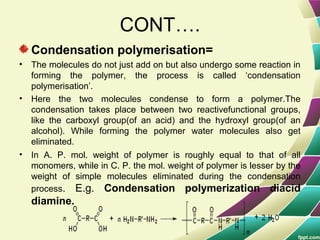
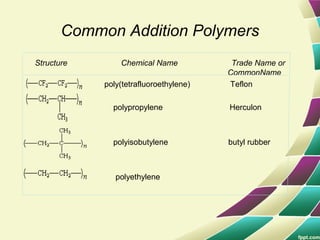
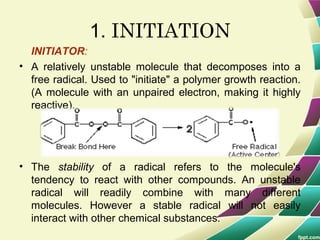
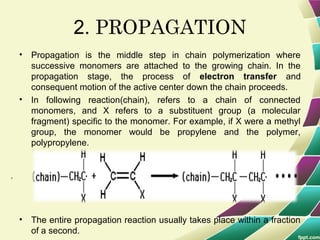
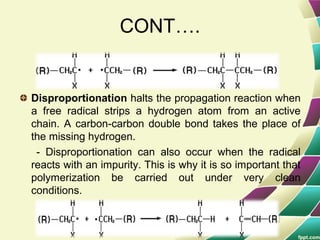
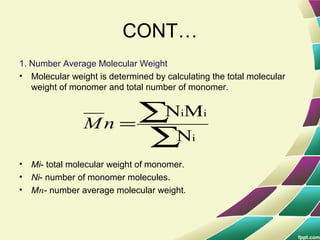
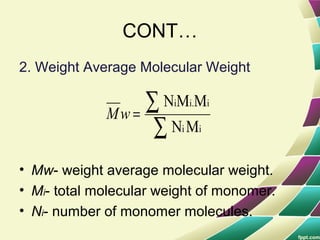
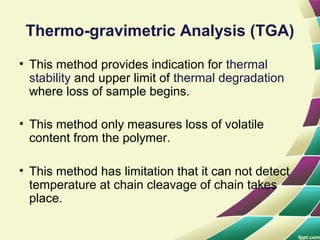
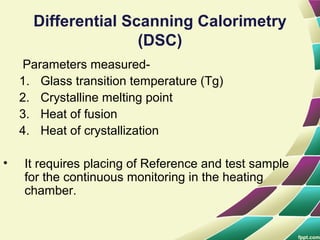
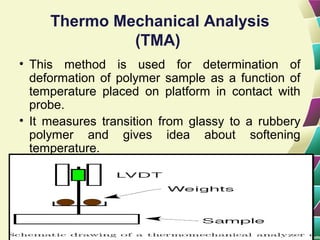
Polymers are large molecules formed by combining many smaller molecules called monomers. They are made through polymerization reactions where monomers join together in chains. There are two main types of polymerization - addition and condensation. Polymers have a wide variety of applications including plastics, fibers, elastomers and more. Their properties depend on factors like molecular structure and weight. Thermal analysis techniques are used to characterize polymers and determine properties like glass transition temperature. Biodegradable polymers break down over time and have applications in drug delivery.