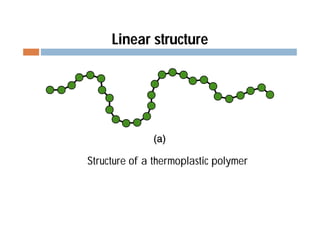
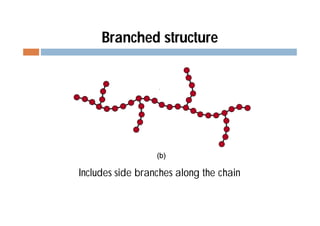
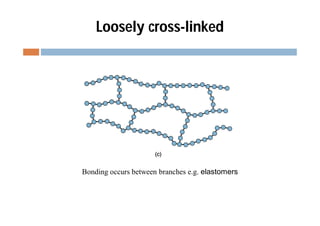
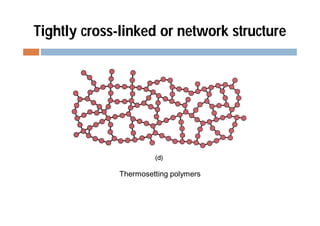
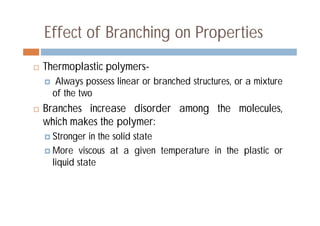
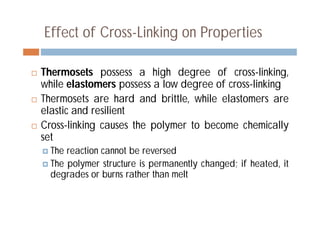
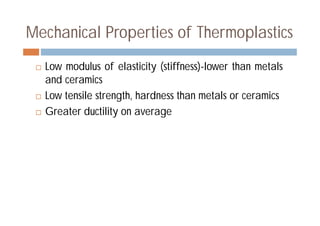
This document discusses different types of polymer structures - linear, branched, and cross-linked. It provides examples of each type and how they relate to polymer properties. Linear structures are characteristic of thermoplastic polymers. Branched structures can also be found in thermoplastics. Cross-linked structures include loosely cross-linked elastomers and tightly cross-linked thermosets. The degree of branching and cross-linking affects properties like strength, viscosity, and whether the polymer is hard/brittle or elastic.



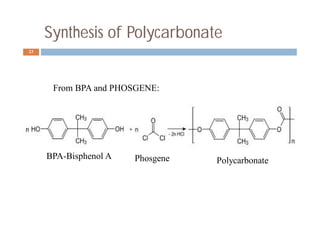
![Synthesis of Polycarbonate
Alternative Route24
From BPA and diphenyl carbonate:
(HOC6H4)2CMe2 + (C6H5O)2CO → -[OC(OC6H4)2CMe2]-n
Diphenyl carbonate Polycarbonate
+
Bisphenol A (BPA)
+ 2 C6H5OH](https://image.slidesharecdn.com/polymers-150621091009-lva1-app6891/85/Polymers-24-320.jpg)