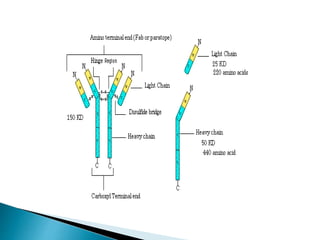
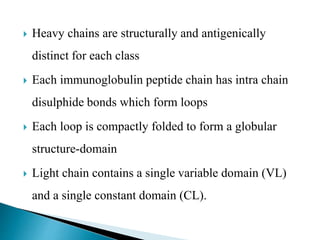
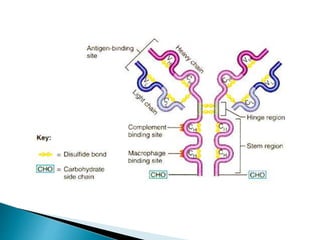
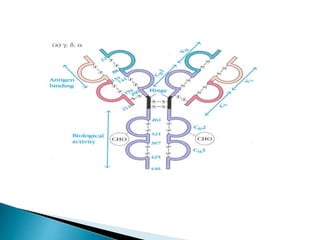
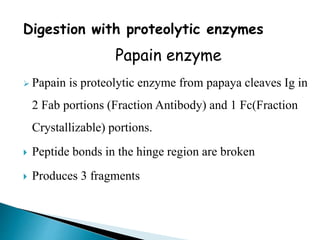
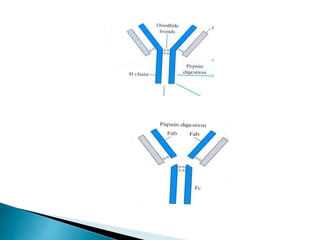
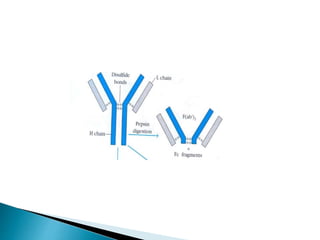
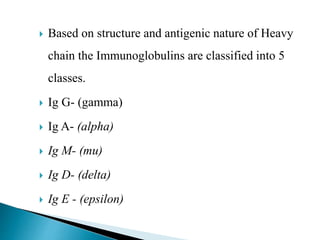
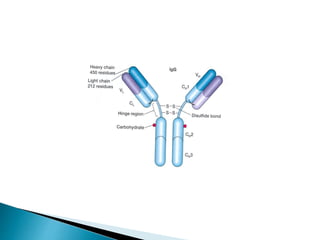
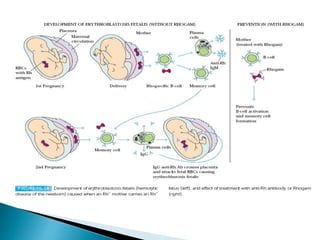
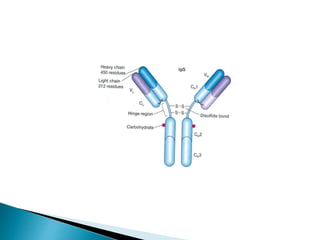
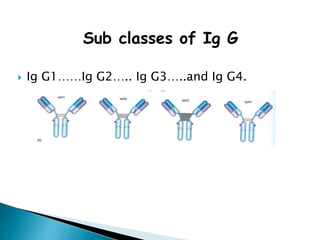
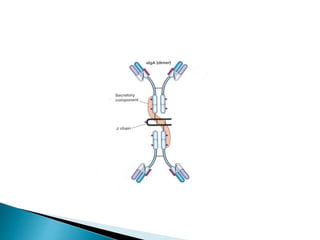
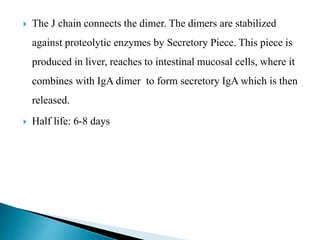
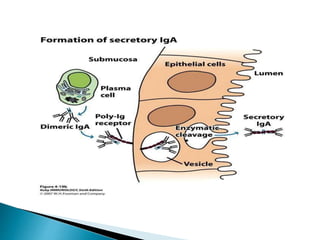
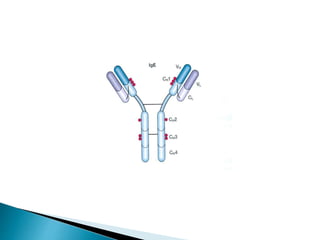
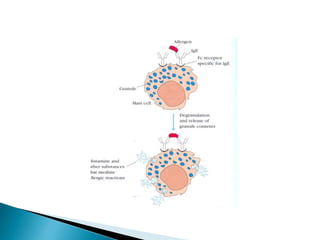
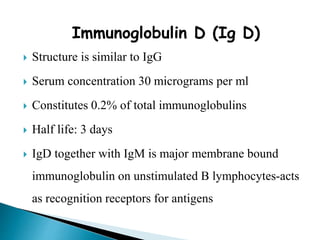
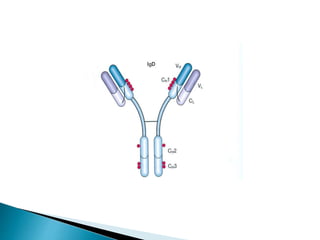
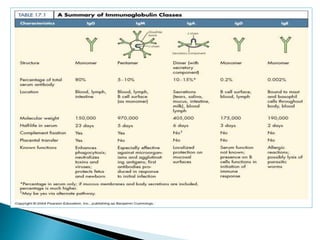
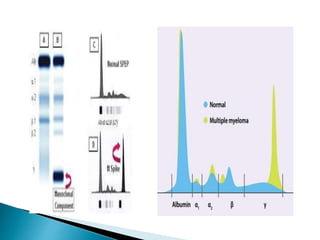
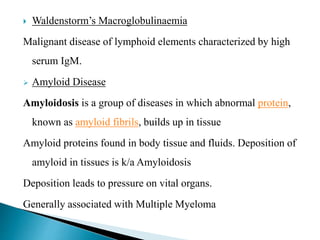
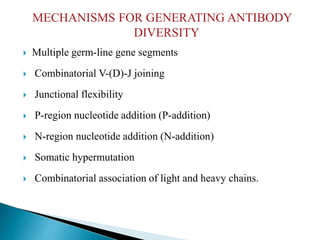
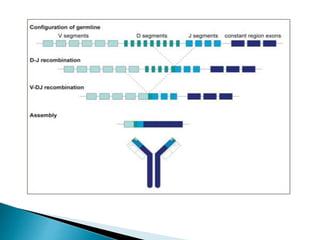
Immunoglobulins, also known as antibodies, are glycoproteins produced by plasma cells that recognize and bind to specific antigens. There are five main classes of immunoglobulins - IgG, IgA, IgM, IgD, and IgE - which differ in their structure and function. IgG is the most abundant antibody found in serum and body tissues, while IgA is predominantly found in secretions such as breast milk, tears, and saliva where it provides immune protection of mucosal surfaces. IgM is the first antibody to respond to new antigens and plays a key role in activating the complement system.