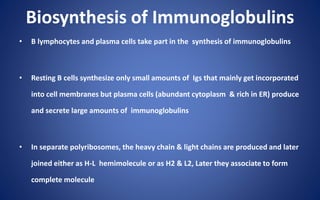
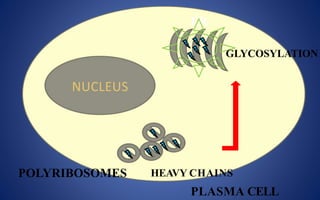
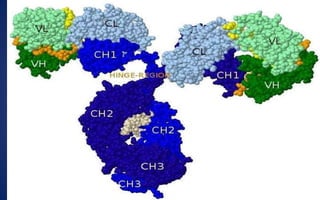
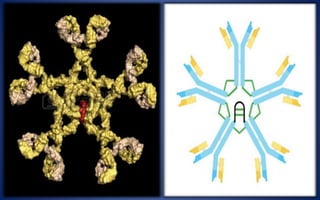
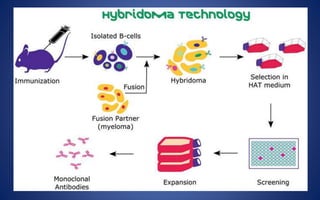
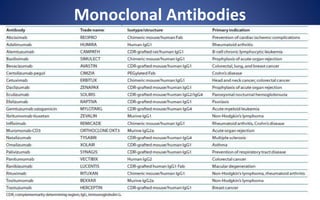
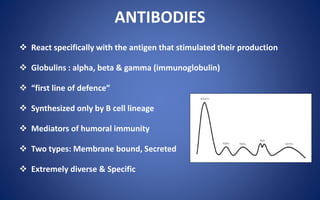
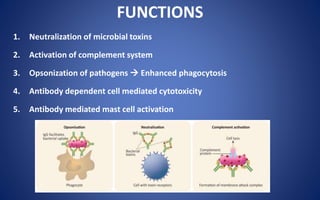
This document discusses immunoglobulins (antibodies), including their five classes (IgG, IgM, IgA, IgE, IgD), structure, functions, and production. The key points are:
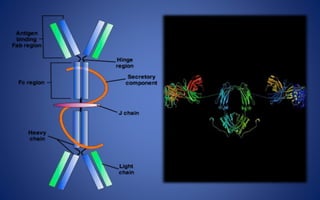
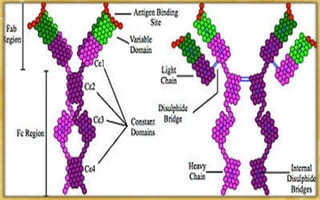
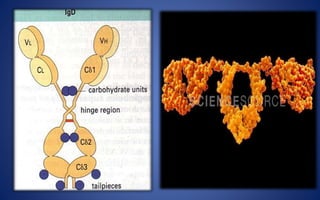
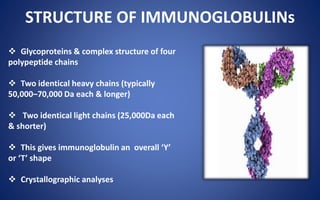
- Immunoglobulins are Y-shaped glycoproteins produced by plasma cells that recognize and bind to specific antigens. They have two heavy chains and two light chains.
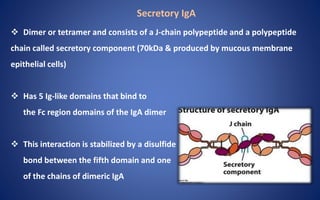
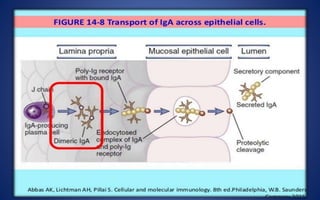
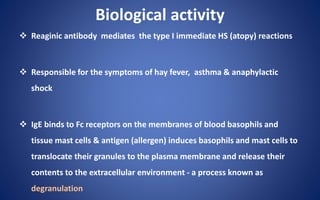
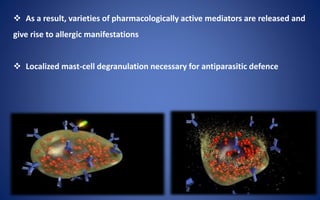
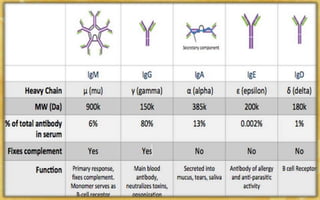
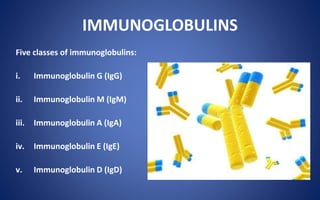
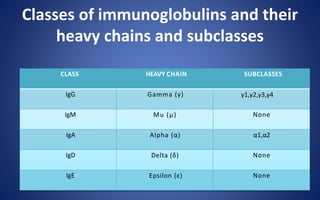
- The five classes of immunoglobulins are IgG, IgM, IgA, IgE, and IgD, which have different structures, functions, and roles in the immune response.
- IgG is the most abundant immunoglobulin and provides long-term protection against pathogens. IgM is the first

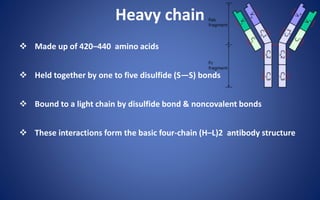
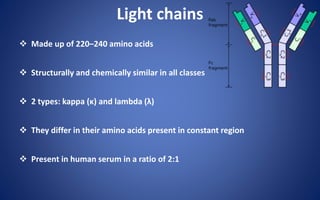
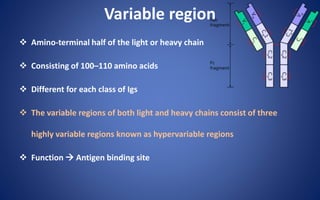
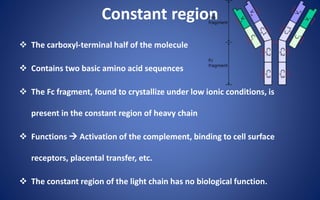
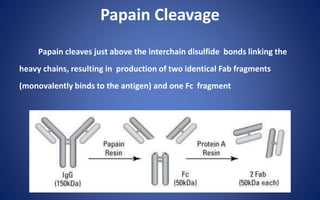
![Pepsin Cleavage
Pepsin cleaves just below these bonds, thereby generating different
digestion products producing an Fc fragment and two Fab fragments
[F(ab)2], which upon exposure to reducing conditions are separated into
Fab monomeric units](https://image.slidesharecdn.com/immunoglobulin-191026204515/85/Immunoglobulin-14-320.jpg)