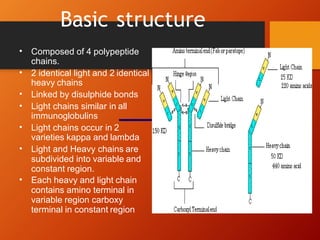
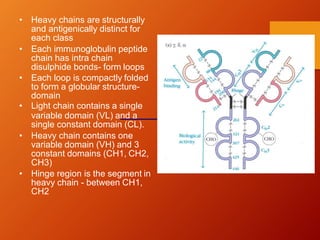
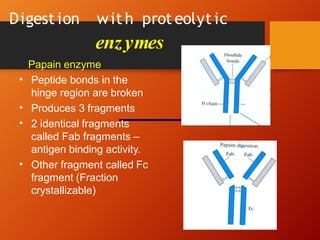
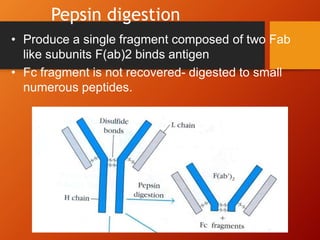
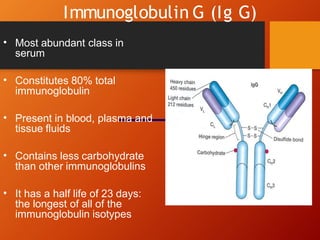
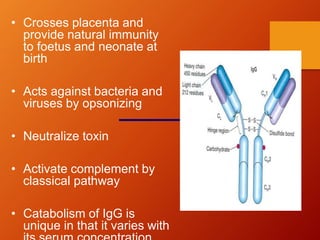
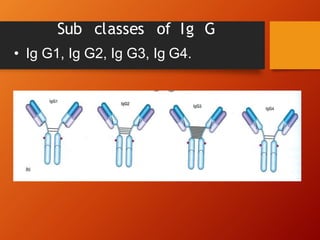
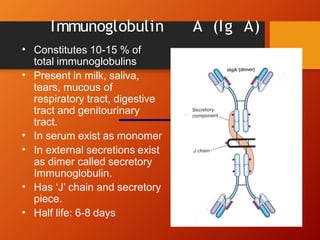
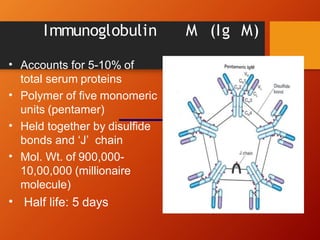
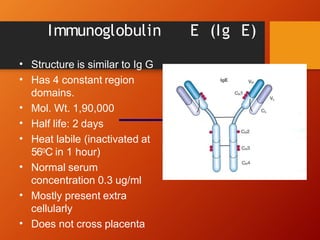
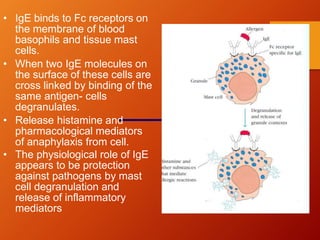
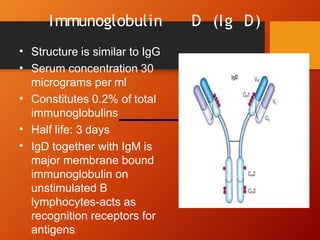
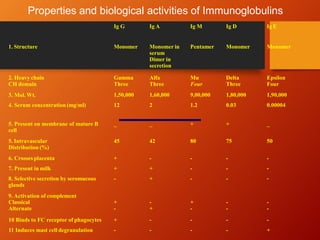
The document provides an overview of immunoglobulins, explaining their structure, classes, and biological functions. It details the composition of immunoglobulins, their roles in immune response, and specific characteristics of each class (IgG, IgA, IgM, IgE, IgD). The document also emphasizes the unique functions and interactions of these immunoglobulins in providing immunity and reinforcing defense against pathogens.