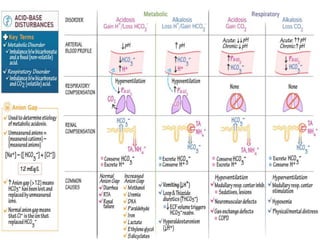
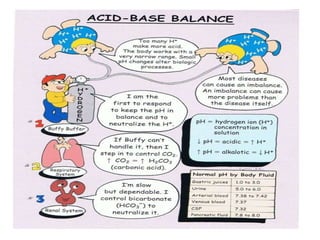
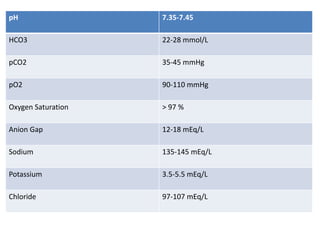
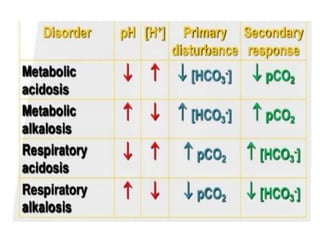
This document discusses acid-base balance and acid-base disorders. It defines the anion gap and explains how to calculate it. It describes the causes and management of different types of acid-base disorders including metabolic acidosis, respiratory acidosis, metabolic alkalosis and respiratory alkalosis. Compensation mechanisms for each disorder are explained. Normal ranges for pH, HCO3, pCO2, pO2 and other electrolytes are provided. Multiple choice questions related to acid-base disorders are included at the end.

![ANION GAP
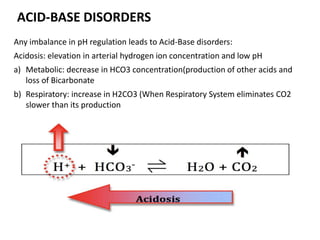
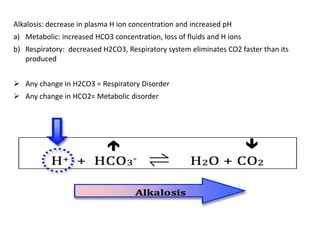
● The difference between the sums of the concentrations of the
measured cations (Na+ and K+) and that of measured anions (Cl–
and HCO3
–) is known as the anion gap. Thus, the anion gap
represents unmeasured anions in the plasma. It can be calculated
as follows:
Anion gap (A–) = ([Na+] + [K+]) – ([Cl–] + [HCO3
–])
= (136 + 4)– (100 + 25)
A– = 15 mEq/L
(Continued…](https://image.slidesharecdn.com/acid-basebalance-2-200414145002/85/Acid-base-balance-2-3-320.jpg)