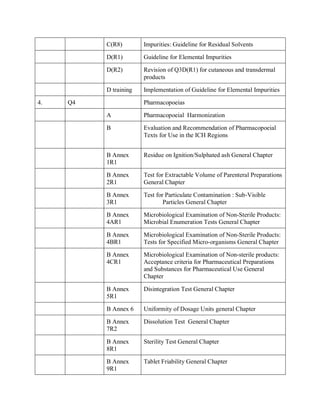
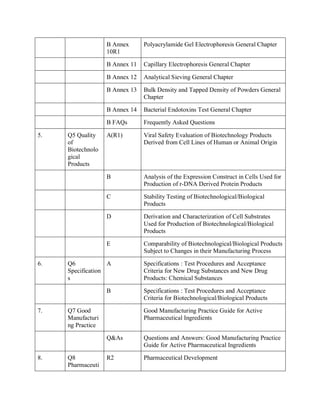
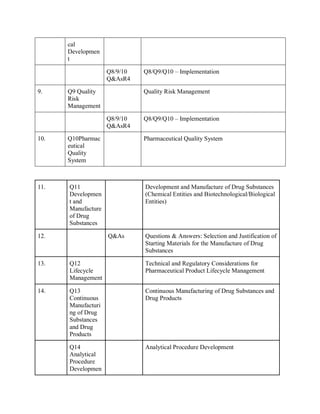
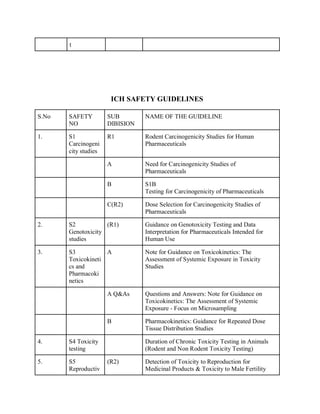
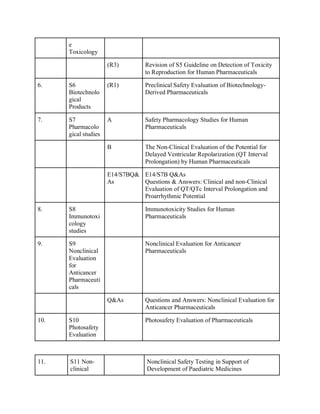
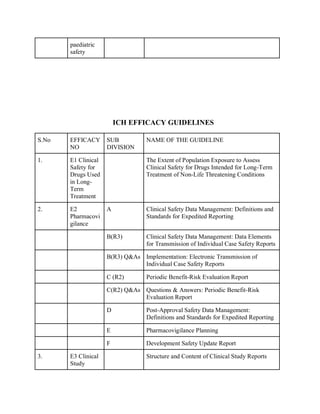
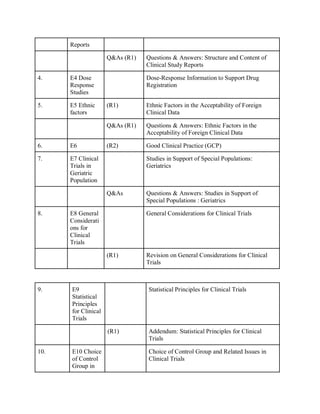
The document discusses the International Council for Harmonisation (ICH) guidelines related to pharmaceutical quality assurance, covering areas such as stability testing, impurities, good manufacturing practices, and clinical trial safety. It details the various categories and specific guidelines within quality, safety, and efficacy to ensure the safety, quality, and efficacy of medicines. Furthermore, it outlines essential considerations for regulatory compliance and risk management in the pharmaceutical industry.