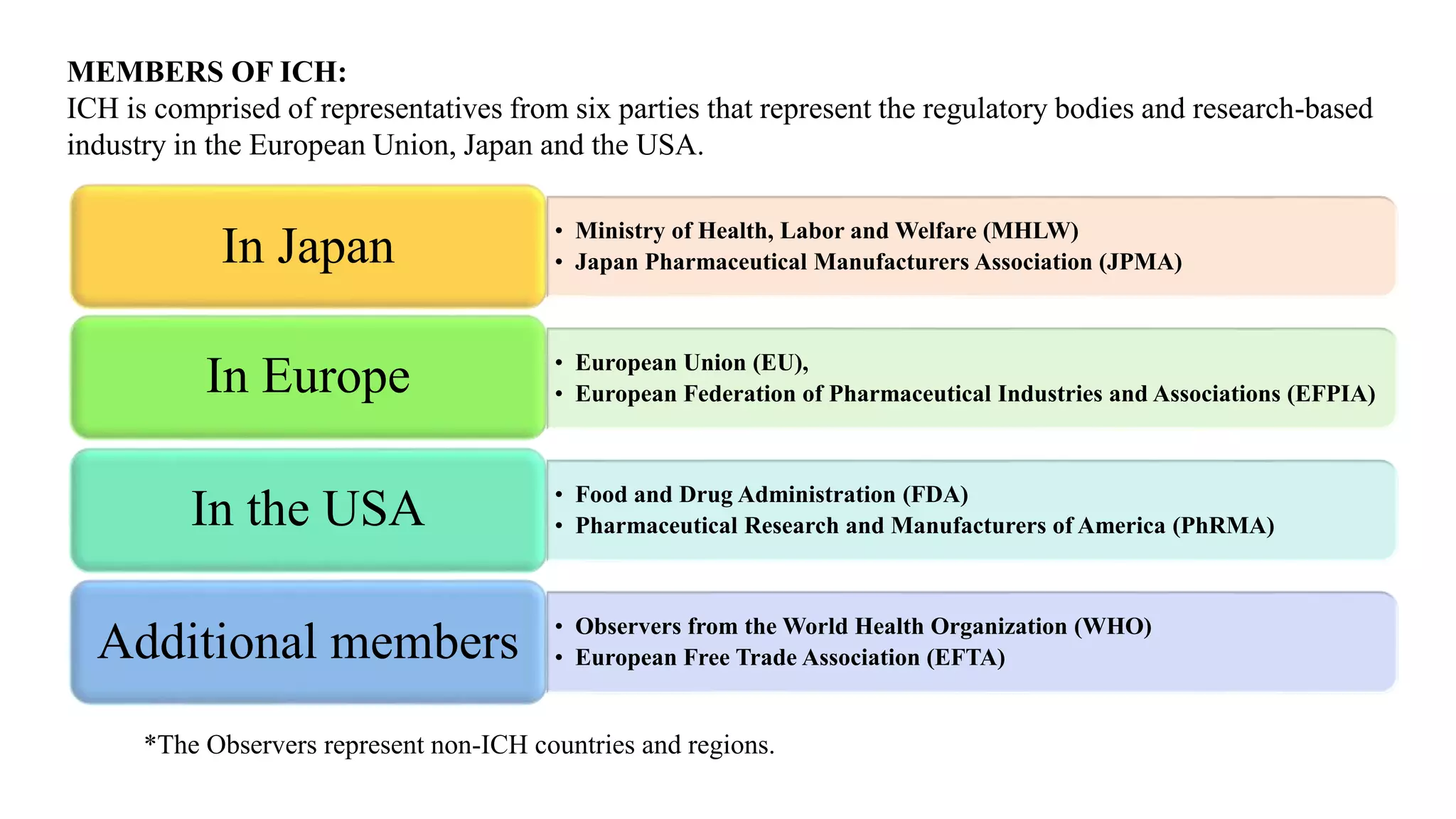
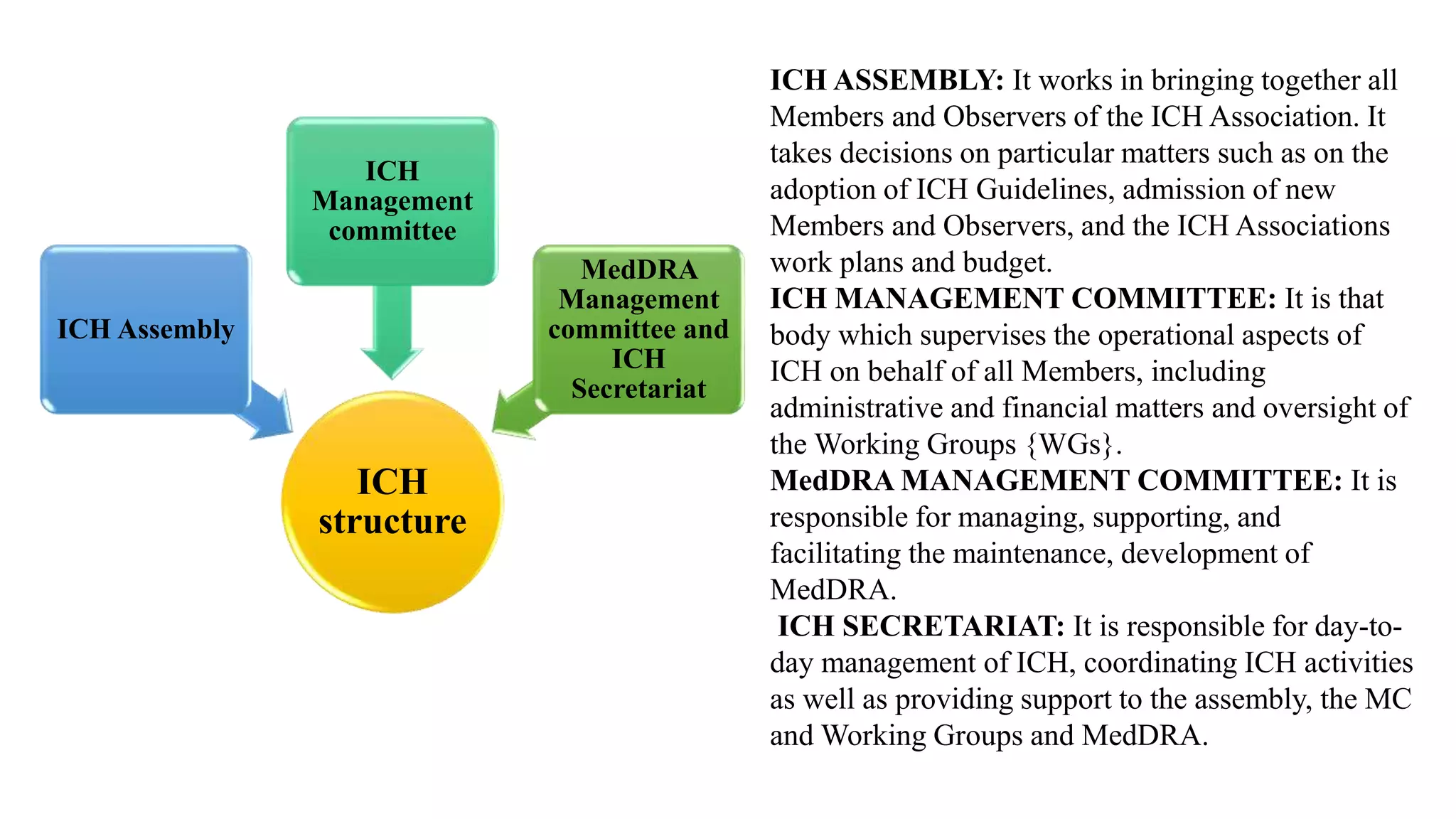
The document provides information on the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). The ICH aims to harmonize technical requirements for drug registration among countries in Europe, Japan, and the United States. It seeks to increase international harmonization, ensure safety and efficacy of medicines, and develop pharmaceuticals efficiently. The ICH provides guidelines on drug quality, efficacy, and safety. It covers topics like stability testing, analytical validation, impurities, biotechnology products, specifications, good manufacturing practices, quality risk management, and quality systems.