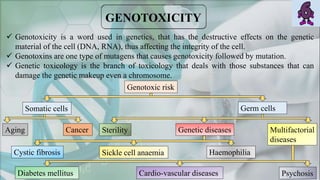
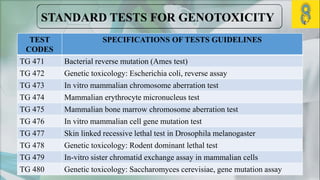
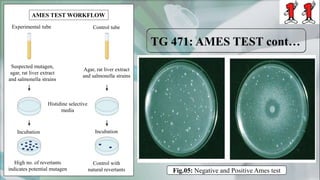
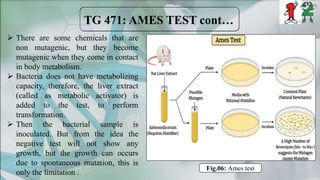
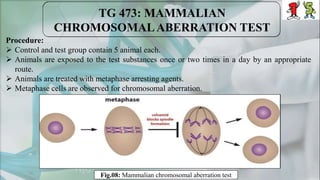
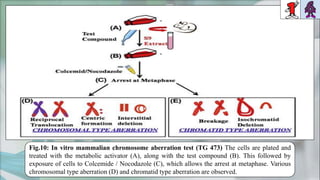
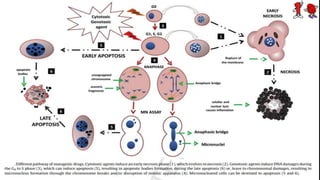
The document presents a genotoxicity study covering essential genetic concepts such as genes, alleles, genotypes, and their role in health risks like cancer and genetic diseases. It details various testing methodologies including the Ames test, comet assay, and chromosomal aberration tests, aligned with international guidelines for assessing genotoxicity. The study emphasizes the need for modern testing approaches due to limitations of traditional methods and highlights ongoing research and advancements in the field.