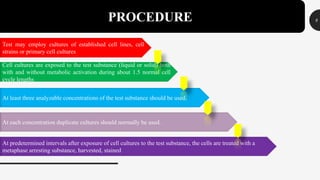
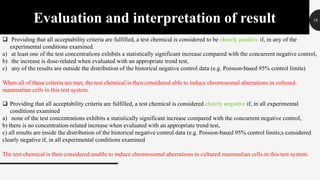
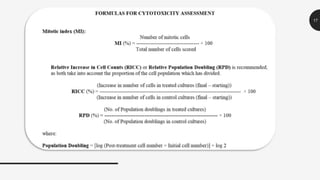
This document provides guidelines for conducting an in vitro mammalian chromosomal aberration test to identify substances that cause structural chromosomal aberrations. The test involves exposing cultured mammalian cells to test substances both with and without metabolic activation. Cells are harvested and analyzed microscopically for chromosomal aberrations like breaks and exchanges. Acceptability criteria include using established cell lines or primary cultures and scoring an adequate number of metaphase cells. A test substance is considered positive if it causes a statistically significant, dose-dependent increase in structural chromosomal aberrations compared to negative controls.
![WELCOME
TO BSBT 105
OCED Guideline for the testing of Chemicals
[473]-In Vitro Mammalian Chromosomal Aberration Test
Submitted to:
Dr. M.S.Vyas
FUNDAMENTALS OF TOXICOLOGY](https://image.slidesharecdn.com/oced473-chromosomalaberration-200420105836/85/Oced-473-chromosomal-aberration-1-320.jpg)